��Ŀ����
 ��2013?��Ԫ���ʼ죩��Ԫ�ض��������������������Ҫ���壮
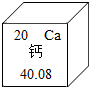
��2013?��Ԫ���ʼ죩��Ԫ�ض��������������������Ҫ���壮��1����Ԫ�����ڱ��У���Ԫ�ص���Ϣ��ͼ��ʾ����Ԫ��ԭ�ӵĺ˵����Ϊ
20
20
����2����ͯȱ�ƿ��ܻᵼ��
���Ͳ�
���Ͳ�
���ƶѪ֢�������Ͳ���������3������������ˮ��Ӧ���˷�Ӧ������
ABC
ABC
������ĸ��ţ���A������ˮ�� B����Ca��OH��2 C������ʳ��
��4���������Ƶ��׳���
��ʯ�һ���ʯ��
��ʯ�һ���ʯ��
����ˮ��Һ��̼���Ʒ�Ӧ���Ʊ��ռ��Ӧ�Ļ�ѧ����ʽΪNa2CO3+Ca��OH��2=CaCO3��+2NaOH
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
����������1������Ԫ�����ڱ��и�Ԫ�ص���Ϣͼ������
��2������������ȱ�ƻ�����Ͳ��ͷ������������Ծݴ˽��
��3�������ƺ�ˮ��Ӧ�ų��������ȣ����Ծݴ˽��
��4�����������׳���ʯ�һ���ʯ�ң��������ƺ�̼���Ʒ�Ӧ�����������ƺ�̼��ƣ����Ծݴ˽����⣮
��2������������ȱ�ƻ�����Ͳ��ͷ������������Ծݴ˽��
��3�������ƺ�ˮ��Ӧ�ų��������ȣ����Ծݴ˽��
��4�����������׳���ʯ�һ���ʯ�ң��������ƺ�̼���Ʒ�Ӧ�����������ƺ�̼��ƣ����Ծݴ˽����⣮
����⣺��1������Ԫ�����ڱ��и�Ԫ�ص���Ϣͼ��֪����Ԫ��ԭ�ӵĺ˵����Ϊ=ԭ������=20��
��2������������ȱ�ƻ�����Ͳ��ͷ����������ʴ�Ϊ�����Ͳ���
��3�������ƺ�ˮ��Ӧ�ų��������ȣ�������Ϊ�����������ˮ�֣������ռ�������������ܹ���ʳ����м��ȣ��÷�Ӧ����������ȡ��ʯ�ң���ѡABC��
��4�����������׳���ʯ�һ���ʯ�ң��������ƺ�̼���Ʒ�Ӧ�����������ƺ�̼��ƣ��䷴Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH���ʴ�Ϊ����ʯ�һ���ʯ�ң�Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
��2������������ȱ�ƻ�����Ͳ��ͷ����������ʴ�Ϊ�����Ͳ���
��3�������ƺ�ˮ��Ӧ�ų��������ȣ�������Ϊ�����������ˮ�֣������ռ�������������ܹ���ʳ����м��ȣ��÷�Ӧ����������ȡ��ʯ�ң���ѡABC��
��4�����������׳���ʯ�һ���ʯ�ң��������ƺ�̼���Ʒ�Ӧ�����������ƺ�̼��ƣ��䷴Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH���ʴ�Ϊ����ʯ�һ���ʯ�ң�Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
�����������ѶȲ�����������Ԫ�����ڱ�������⣬������ʯ�ҵ������Լ���ѧʽ����д�ȣ�

��ϰ��ϵ�д�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
�����Ŀ
 ��2013?��Ԫ���ʼ죩��ͼΪij���ӵĽṹʾ��ͼ����������ǣ�������
��2013?��Ԫ���ʼ죩��ͼΪij���ӵĽṹʾ��ͼ����������ǣ�������