��Ŀ����
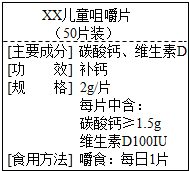 ��ͼΪ��XX����Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������и��⣮
��ͼΪ��XX����Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������и��⣮��1��ÿƬ��Ƭ�����ٺ���Ԫ�ص�����Ϊ
��2��С��ͬѧΪ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��10Ƭ��Ƭ����������С�ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����30g������С�ձ���ʣ�����ʵ�������43.4g�������ձ��������ٶ���Ƭ�����ɷֲ������ᷴӦ����
�����ɵĶ�����̼������Ϊ
��ͨ�������жϸø�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
��������1������ÿƬ������Ϊ2g��ÿƬ��̼��Ƶĺ�����1.5g������̼����иƵ��������������㺬�Ƶ�������
��2������Ʒ�Ӧ��ֻ�ж�����̼�����壬��ɸ����������غ����������ɶ�����̼��������
�ڸ��ݶ�����̼����������̼��ƺ����ᷴӦ�Ļ�ѧ��Ӧ����ʽ������̼��Ƶ���������̼��Ƶ�������1.5g����ʵ����֮�Ͳ���ʵ��
��2������Ʒ�Ӧ��ֻ�ж�����̼�����壬��ɸ����������غ����������ɶ�����̼��������
�ڸ��ݶ�����̼����������̼��ƺ����ᷴӦ�Ļ�ѧ��Ӧ����ʽ������̼��Ƶ���������̼��Ƶ�������1.5g����ʵ����֮�Ͳ���ʵ��
����⣺��1��̼����иƵ���������Ϊ
��100%=40%��
��ÿƬ��̼��Ƶĺ�����1.5g��
��Ƭ��Ƭ�����ٺ���Ԫ�ص�����Ϊ1.5g��40%=0.6g���ʴ�Ϊ��0.6��
��2������ÿƬ������Ϊ2g��10Ƭ��Ƭ������Ϊ2g��10=20g��
�����������غ��֪��
������̼������Ϊ20g+30g-43.4g=6.6g���ʴ�Ϊ��6.6��
����10Ƭ��Ƭ��̼��Ƶ�����Ϊxg����
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 6.6g
=
���x=15g��
��ÿƬ��̼��Ƶ�����Ϊ
=1.5g��
���Ƭ��Ʒ��ǩͼ�еĹ���б��ÿƬ��̼��Ƶĺ�����1.5g��
����ʵ��
�𣺸ø�Ƭ��̼��Ƶĺ�����ע��ʵ��
| 40 |
| 40+12+16��3 |
��ÿƬ��̼��Ƶĺ�����1.5g��
��Ƭ��Ƭ�����ٺ���Ԫ�ص�����Ϊ1.5g��40%=0.6g���ʴ�Ϊ��0.6��
��2������ÿƬ������Ϊ2g��10Ƭ��Ƭ������Ϊ2g��10=20g��
�����������غ��֪��
������̼������Ϊ20g+30g-43.4g=6.6g���ʴ�Ϊ��6.6��
����10Ƭ��Ƭ��̼��Ƶ�����Ϊxg����
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 6.6g
| 100 |
| x |
| 44 |
| 6.6g |
���x=15g��
��ÿƬ��̼��Ƶ�����Ϊ
| 15g |
| 10 |
���Ƭ��Ʒ��ǩͼ�еĹ���б��ÿƬ��̼��Ƶĺ�����1.5g��
����ʵ��
�𣺸ø�Ƭ��̼��Ƶĺ�����ע��ʵ��
��������������ϢΪ����������ѧ���������Ⲣ��������������ͬʱ����ѧ�����������Ϣ���л�ѧ��Ӧ����ʽ�ļ��㣬ע���˻�ѧ���������ϵ��Ҳ���Ժ�ϰ�����Ҫ���鷽��

��ϰ��ϵ�д�
�����Ŀ
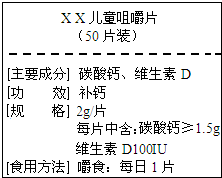 ����ͼΪ��XX����Ƭ��Ʒ��ǩͼ������ݱ����й���Ϣ������и��⣮
����ͼΪ��XX����Ƭ��Ʒ��ǩͼ������ݱ����й���Ϣ������и��⣮ ����ͼΪ��XX����Ƭ��Ʒ��ǩͼ������ݱ����й���Ϣ������и��⣮
����ͼΪ��XX����Ƭ��Ʒ��ǩͼ������ݱ����й���Ϣ������и��⣮
