题目内容
| 某同学为探究铜铁合金中铁的质量分数,先后进行了三次实验,实验数据见下表: | ||||||||||||||||
| ||||||||||||||||
| 根据该同学的实验,回答下列问题:((2)(3)要求写出计算过程) (1)上表三次实验中,有一次恰好完全反应,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是 _________ g. (2)该合金中铁的质量分数是多少? (3)第三次实验所得溶液的溶质质量分数为多少?(精确到0.1%) |
| (1)80 (2)设生成0.4克的氢气需要铁为x,生成硫酸亚铁为y, Fe+H2SO4=FeSO4+H2↑ 56 152 2 x y 0.4g 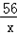 = =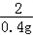 x=11.2g 所以铁的质量分数为: 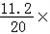 100%=56%, 100%=56%,答:该合金中铁的质量分数是:56%; (3)设生成硫酸亚铁为y, Fe+H2SO4=FeSO4+H2↑ 56 152 2 x y 0.4g 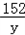 = =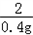 y=30.4g 反应后的溶液质量为:80+11.2﹣0.4=90.8g, 所以反应后的溶质质量分数为: 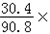 100%=33.5%. 100%=33.5%.答:(3)第三次实验所得溶液的溶质质量分数为:33.5%. |

练习册系列答案
相关题目
某同学为探究铜铁合金中铁的质量分数,先后进行了三次实验,实验数据见下表:
根据该同学的实验,回答下列问题:((2)(3)要求写出计算过程)
(1)上表三次实验中,有一次恰好完全反应,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是 g.
(2)该合金中铁的质量分数是多少?
(3)第三次实验所得溶液的溶质质量分数为多少?(精确到0.1%)
| 第一次 | 第二次 | 第三次 | |
| 所取合金的质量/g | 20 | 20 | 20 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
(1)上表三次实验中,有一次恰好完全反应,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是
(2)该合金中铁的质量分数是多少?
(3)第三次实验所得溶液的溶质质量分数为多少?(精确到0.1%)
某同学为探究铜铁合金中铁的质量分数,先后进行了三次实验,实验数据见下表:
根据该同学的实验,回答下列问题:((2)(3)要求写出计算过程)
(1)上表三次实验中,有一次恰好完全反应,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______ g.
(2)该合金中铁的质量分数是多少?
(3)第三次实验所得溶液的溶质质量分数为多少?(精确到0.1%)
| 第一次 | 第二次 | 第三次 | |
| 所取合金的质量/g | 20 | 20 | 20 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
(1)上表三次实验中,有一次恰好完全反应,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______ g.
(2)该合金中铁的质量分数是多少?
(3)第三次实验所得溶液的溶质质量分数为多少?(精确到0.1%)
