��Ŀ����
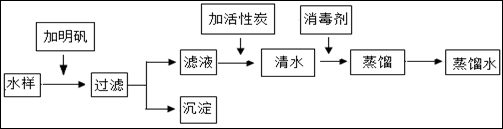
��6�֣���Ȼ���е�ˮ������������ԺͲ��������ʣ�ͨ������;������ʹˮ�õ���ͬ�̶ȵľ�������ͼ������ˮ������ˮ����ʾ��ͼ��

��1����������ˮ�ķ����У��������������������������������ȡ�
��2��Ͷҩ������Ŀ����ɱ��ˮ�е�ϸ���������ͼ����档����ˮ�����ö������ȣ�ClO2������������������Ԫ�صĻ��ϼ�Ϊ ��������й�ҵ�ƶ������ȵĻ�ѧ��Ӧ����ʽ��Cl2 + 2 NaClO2 ="=" 2 ClO2 + ��
��3������Ӧ����ϧÿһ��ˮ���������������ڽ�Լ��ˮ���� ��
��4������ˮ�к�������Ca(HCO3)2�ȿ����Ի������ˮʱCa(HCO3)2�����ֽⷴӦ�����������Ե�̼��ơ�ˮ�Ͷ�����̼������Ǻ��г���ˮ����ԭ��֮һ����д������Ca(HCO3)2���ȷֽ�Ļ�ѧ����ʽ ��

��1����������ˮ�ķ����У��������������������������������ȡ�
��2��Ͷҩ������Ŀ����ɱ��ˮ�е�ϸ���������ͼ����档����ˮ�����ö������ȣ�ClO2������������������Ԫ�صĻ��ϼ�Ϊ ��������й�ҵ�ƶ������ȵĻ�ѧ��Ӧ����ʽ��Cl2 + 2 NaClO2 ="=" 2 ClO2 + ��
��3������Ӧ����ϧÿһ��ˮ���������������ڽ�Լ��ˮ���� ��
| A��ϴ�˵�ˮ�������� | B��δ����Ŀ�Ȫˮ���ֵ��� |
| C���ò���ϵ���ˮ��ϴ��� | D��ϴ���ԡҺʱ������ˮ��ͷ |
��1������ ���� ��2��+4 2NaCl ��3��BC
��4��Ca(HCO3)2 CaCO3+H2O+CO2��
CaCO3+H2O+CO2��
��4��Ca(HCO3)2
 CaCO3+H2O+CO2��
CaCO3+H2O+CO2�����⿼�����ˮ�ľ�����ˮ��Դ����Ⱦ����Σ���д��ѧ����ʽ��
��1���۲쾻ˮ����ʾ��ͼ��֪����ˮ�������й��ˡ�������
��2���ڻ������л��ϼ��ܺ���0�����������У���Ԫ����-2�ۣ�������Ԫ�صĻ��ϼ���+4�ۣ���ѧ��Ӧǰ��Ԫ�ص������ԭ�Ӹ������䣬��ѧ��Ӧǰ����Ԫ�ء���Ԫ�غ���Ԫ�أ��ҷ�Ӧǰ��4����ԭ�ӣ�2����ԭ�ӣ�4����ԭ�ӣ����Ըÿ�ӦΪ2NaCl��
��3�������п�ʹ�ý�ˮ��ͷ��ϴ��ˮ�����Ƚ��н�Լˮ����ȻBC�����Ͻ�ˮԭ��ѡBC��
��4��̼��������ȷֽ����������Ե�̼��ơ�ˮ�Ͷ�����̼����Ӧ�ķ���ʽ��Ca(HCO3)2 CaCO3+H2O+CO2��
CaCO3+H2O+CO2��
��1���۲쾻ˮ����ʾ��ͼ��֪����ˮ�������й��ˡ�������
��2���ڻ������л��ϼ��ܺ���0�����������У���Ԫ����-2�ۣ�������Ԫ�صĻ��ϼ���+4�ۣ���ѧ��Ӧǰ��Ԫ�ص������ԭ�Ӹ������䣬��ѧ��Ӧǰ����Ԫ�ء���Ԫ�غ���Ԫ�أ��ҷ�Ӧǰ��4����ԭ�ӣ�2����ԭ�ӣ�4����ԭ�ӣ����Ըÿ�ӦΪ2NaCl��
��3�������п�ʹ�ý�ˮ��ͷ��ϴ��ˮ�����Ƚ��н�Լˮ����ȻBC�����Ͻ�ˮԭ��ѡBC��
��4��̼��������ȷֽ����������Ե�̼��ơ�ˮ�Ͷ�����̼����Ӧ�ķ���ʽ��Ca(HCO3)2
 CaCO3+H2O+CO2��
CaCO3+H2O+CO2��
��ϰ��ϵ�д�
�����Ŀ