��Ŀ����
��ѧ��ȤС��ⶨijʯ��ʯ��Ʒ��̼��Ƶ������������ٶ���ʯ��ʯ��Ʒ�г�̼����⣬�������ʲ���ϡ���ᷴӦҲ������ˮ����I ���ǵķ����ǣ���1��ȡ3.0g��Ʒ�����ձ���
��2������������ϡ������ǡ�ò��ٲ�������ʱ���ⶨ��Ӧ�����ɵ�CO2������
��3������CO2�����������Ʒ��̼��Ƶ�����������������
II Ϊ�ⶨCO2��������������������·�������������������Ʒ�����
��1��ѡ����ͼ______����ס����ҡ���װ�ÿɲ���������CO2��______�������ô�ʱCO2���ܶȣ��ɼ���CO2��������ͼ��ƿ��ˮ�����Ͳ��������______��
��2������ѡ�ø÷���ʱ�����������ڳ��������Ϊ440mL����֪������CO2������ܶ�Ϊ2.0g/L����������Ӧ�ų����������Ϊ______g������ݻ�ѧ��Ӧ����ʽ�����ʯ��ʯ��Ʒ��CaCO3������������
III��ʵ���С������ò�ͬ�ķ����ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������
��1��ȡmg��Ʒ�����ձ���
��2������������ϡ���������ٲ�������ʱ�����ˣ�ϴ�ӡ���������ʣ���������Ϊng��
��3������ʣ���������������Ʒ��̼��Ƶ���������Ϊ______������m��n�Ĵ���ʽ��ʾ��
������������ʵ�鷽���⣬�����Բ��õķ����ǣ�______��������Ӧǰ�������������ļ�С�������ݼ�С�������Ʒ��̼��Ƶ�����������������

���𰸡���������1�����ݶ�����̼��������ѡ����װ�õ��ص�����ɸ���Ľ��
��2�������ܶȹ�ʽ���������������̼�����������Ȼ����̼�����ϡ���ᷴӦ�Ļ�ѧ����ʽ�������̼��Ƶ����������������̼��Ƶ�����������
���������غ㶨�ɿ�����ɸ���Ľ��
��������̼��Ƶ���������ɽ��
����⣺��1��������̼�ܹ�����ˮ������Ϊ��ʹʵ������ȷ��������̼���ܺ�ˮ�Ӵ�������Ӧ��ѡ���װ�ã�������Ͳ�е�ˮ�������Ϊ������̼���������װ�����͵����þ���Ϊ�˷�ֹ������̼����ˮ��Ӱ��ʵ�����ģ�
��2�����������֪���ɶ�����̼��������Ϊ440mL=0.44L�����Ը����ܶȹ�ʽ����֪�����ɶ�����̼���������Ϊ��m=��V=2.0g/L×0.44L=0.88g��
��ʯ��ʯ��Ʒ��CaCO3������Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 0.88g
 =
=
��ã�x=2.0g
��ʯ��ʯ��Ʒ��CaCO3����������Ϊ�� ×100%=66.7%��
×100%=66.7%��
�𣺸�ʯ��ʯ��Ʒ��CaCO3����������Ϊ66.7%��
���������غ㶨�ɿ���֪������Ӧǰ�������������ٵ�������Ϊ����Ӧ�˵�̼��Ƶ���������Ϊ����m-n��g�����Կ��Ա�ʾ����Ʒ��̼��Ƶ���������Ϊ�� ×100%��
×100%��
����̼����ڸ������ܹ��ֽ����������̼�������ƣ����Կ��Խ�ʯ��ʯ�������գ���������ʯ��ʯ��Ʒ��̼��Ƶ�����������
�ʴ�Ϊ����1���ף��������ֹ������̼����ˮ��
��2��0.88��66.7%��
�� ×100%��
×100%��
��������������Ʒ��
�������������ն�����̼��̼��ƵĻ�ѧ���ʣ�����ס��Ӧ�Ļ�ѧ����ʽ���ܹ����ݻ�ѧ����ʽ���мļ��㣬����Ҫ�������������Ĺ�ʽ��
��2�������ܶȹ�ʽ���������������̼�����������Ȼ����̼�����ϡ���ᷴӦ�Ļ�ѧ����ʽ�������̼��Ƶ����������������̼��Ƶ�����������
���������غ㶨�ɿ�����ɸ���Ľ��
��������̼��Ƶ���������ɽ��
����⣺��1��������̼�ܹ�����ˮ������Ϊ��ʹʵ������ȷ��������̼���ܺ�ˮ�Ӵ�������Ӧ��ѡ���װ�ã�������Ͳ�е�ˮ�������Ϊ������̼���������װ�����͵����þ���Ϊ�˷�ֹ������̼����ˮ��Ӱ��ʵ�����ģ�
��2�����������֪���ɶ�����̼��������Ϊ440mL=0.44L�����Ը����ܶȹ�ʽ����֪�����ɶ�����̼���������Ϊ��m=��V=2.0g/L×0.44L=0.88g��
��ʯ��ʯ��Ʒ��CaCO3������Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 0.88g
 =
=
��ã�x=2.0g
��ʯ��ʯ��Ʒ��CaCO3����������Ϊ��
 ×100%=66.7%��
×100%=66.7%���𣺸�ʯ��ʯ��Ʒ��CaCO3����������Ϊ66.7%��
���������غ㶨�ɿ���֪������Ӧǰ�������������ٵ�������Ϊ����Ӧ�˵�̼��Ƶ���������Ϊ����m-n��g�����Կ��Ա�ʾ����Ʒ��̼��Ƶ���������Ϊ��
 ×100%��
×100%������̼����ڸ������ܹ��ֽ����������̼�������ƣ����Կ��Խ�ʯ��ʯ�������գ���������ʯ��ʯ��Ʒ��̼��Ƶ�����������
�ʴ�Ϊ����1���ף��������ֹ������̼����ˮ��
��2��0.88��66.7%��
��
 ×100%��
×100%����������������Ʒ��
�������������ն�����̼��̼��ƵĻ�ѧ���ʣ�����ס��Ӧ�Ļ�ѧ����ʽ���ܹ����ݻ�ѧ����ʽ���мļ��㣬����Ҫ�������������Ĺ�ʽ��

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
�����Ŀ
 �������ִ������ҵ������Ӧ�ü�Ϊ�ձ��һ����ϣ�
�������ִ������ҵ������Ӧ�ü�Ϊ�ձ��һ����ϣ���1����֪����ͬ�������£������Ļ��Խǿ���������ᷴӦ�������ݣ����������ٶȾ�Խ�죮Al��Cu��Fe���ֽ�����ϡ������ķ�Ӧ��������ͼ��ʾ��
����ͼ��Y�������Ľ�����
��Al��Cu��Fe���ֽ����Ļ����ǿ������˳��Ϊ
������һ�ֻ��ý���������������ȴ�н�ǿ�Ŀ���ʴ�ԣ���ԭ����
��2�������Ŀ����������������������������ı�־��
���ҹ��Ŵ���¯��ʯ��ZnCO3������ͭ��Cu2O����ľ̿�ۻ�ϼ�����800�����ң����ɵõ���ƽ�������Ƶġ�ҩ�𡱣�
I�����������������Ƶ������Ļƽ�Au������Ϊ
II����ҩ����
���������²����ᡢ�Ӧ�����������ܣ�����Ϊ��δ�����������ɷ��Ѵ�������ȡ�����ѵ���Ҫ���չ������£�
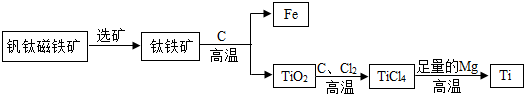
�ڸ�����������Mg��Ӧ�û����ɽ���Ti���÷�Ӧ�Ļ�ѧ����ʽΪ��
�������������еõ��Ľ������л��������������ʣ��ɼ���
��3����֪ij������ĩ�г�����Al�����һ������Fe��Cu��Ϊ֤��Fe��Cu�Ĵ��ڲ��ⶨ����Al������������ij��ѧ��ȤС���ͬѧչ�������µ�ʵ��̽����
�������ߣ�Al������������Һ��Ӧ��������ˮ��ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2Al+2H2O+2NaOH=2NaAlO2+3H2������Fe��Cu��������������Һ��Ӧ��
�������֤��������ĩ�д���Fe��Cu��ʵ����ƣ�
| ʵ����� | ʵ������ | ���� |
| ��ȡ�����Ľ�����ĩ���Թ��У����������� |
����ȥ�� | |
| ���Թܾ��ã���ȥ�ϲ���Һ������������ϡ���ᣮ | ֤�������� | |
| ���Թܾ��ã���ȥ�ϲ���Һ�����ϴ��ʣ����� | ʣ�������Ϻ�ɫ | ֤������ͭ |
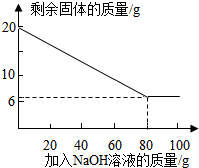 ��Ϊ̽���ý�����ĩ��Al������������ȡ20 g�ý�����ĩ����100 g����������Һƽ���ֳ�5�����μ��룬��ַ�Ӧ���˳����壬����ϴ�ӡ����������ʵ������еõ��IJ���������ͼ�����£�
��Ϊ̽���ý�����ĩ��Al������������ȡ20 g�ý�����ĩ����100 g����������Һƽ���ֳ�5�����μ��룬��ַ�Ӧ���˳����壬����ϴ�ӡ����������ʵ������еõ��IJ���������ͼ�����£�| ��NaOH��Һ�Ĵ��� | ��һ�� | �ڶ��� | ������ | �� |
| ʣ����������/g | 16.5 | n | 9.5 | �� |
�ý�����ĩ��Al����������Ϊ
����ʽ���㣺��������������Һ��������������Ϊ���٣�
 ��2012?��ƽ��һģ���Ȼ��Ƽ�ʯ�Ļ�ѧʽΪKCl?xCaCl2��x��CaCl2��ϵ����������һ�ּطʣ�����ˮ��õ�KCl��CaCl2�Ļ����Һ��ij��ѧ��ȤС��Ϊ�˲ⶨ�Ȼ��Ƽ�ʯ�м�Ԫ�ص�������������ȡ��Ʒ18.55g��ˮ��ȫ�ܽ�õ�KCl��CaCl2�Ļ����Һ210g�������Һ����μ���Na2CO3��Һ�������ij��������Na2CO3��Һ��������ϵ��ͼ��ʾ����֪��Na2CO3+CaCl2�TCaCO3��+2NaCl������㣺
��2012?��ƽ��һģ���Ȼ��Ƽ�ʯ�Ļ�ѧʽΪKCl?xCaCl2��x��CaCl2��ϵ����������һ�ּطʣ�����ˮ��õ�KCl��CaCl2�Ļ����Һ��ij��ѧ��ȤС��Ϊ�˲ⶨ�Ȼ��Ƽ�ʯ�м�Ԫ�ص�������������ȡ��Ʒ18.55g��ˮ��ȫ�ܽ�õ�KCl��CaCl2�Ļ����Һ210g�������Һ����μ���Na2CO3��Һ�������ij��������Na2CO3��Һ��������ϵ��ͼ��ʾ����֪��Na2CO3+CaCl2�TCaCO3��+2NaCl������㣺
 ��2013?������һģ��Ϊ�ⶨij������ʯ��������������������ij��ѧ��ȤС���ͬѧ�ù�����һ����̼��10g������ʯ��Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����������ɵ�������100g����������Һǡ����ȫ���գ��γɲ�������Һ������Һ�������뷴Ӧʱ��ı仯��ϵ��ͼ���Է������
��2013?������һģ��Ϊ�ⶨij������ʯ��������������������ij��ѧ��ȤС���ͬѧ�ù�����һ����̼��10g������ʯ��Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����������ɵ�������100g����������Һǡ����ȫ���գ��γɲ�������Һ������Һ�������뷴Ӧʱ��ı仯��ϵ��ͼ���Է������