��Ŀ����
ij��ҩ��˾����һ�ָ�Ƭ������Ҫ�ɷ���̼��ƣ�Ϊ�ⶨ��Ƭ��̼��Ƶĺ�����ȡ5Ƭ��Ƭ�����ȫ��ת������ƿ�У���������ʵ�飨�����Ƭ��̼���֮��ijɷֲ������ᷴӦ��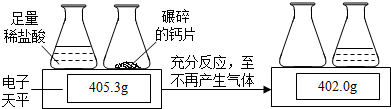
��1�������ʵ�����ݣ����Լ���ÿƬ��Ƭ�к�̼��Ƶ���������д��������̣�ע���ʽ��
��2��С˳ͬѧ��Ϊ�÷����ⶨ�Ķ�����̼��������ƫ�������� С��ͬѧ��Ϊ�÷����ⶨ�Ķ�����̼��������ƫС�������� ��������̼��������Һ���ܽ�ȼ�С���ɺ��Բ��ƣ�

��1�������ʵ�����ݣ����Լ���ÿƬ��Ƭ�к�̼��Ƶ���������д��������̣�ע���ʽ��
��2��С˳ͬѧ��Ϊ�÷����ⶨ�Ķ�����̼��������ƫ��������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1�����������غ㶨�ɣ�ǰ������֮�Ϊ���ɵĶ�����̼�����������ݶ�����̼���������û�ѧ����ʽ����̼��Ƶ�������
��2�����ݷ�Ӧ�����п��ܳ��ֵ��������
��2�����ݷ�Ӧ�����п��ܳ��ֵ��������
����⣺��1������������̼����Ϊ��405.3g-402.0g=3.3g
��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.3g
=
x=7.5g
ÿƬ��Ƭ�к�̼��Ƶ�����Ϊ��7.5g��5=1.5g
��2��С˳ͬѧ��Ϊ�÷����ⶨ�Ķ�����̼��������ƫ��������ˮ�������Ȼ��������ӷ��������У���ɷ�Ӧ�����ʵ�������С��402.0g���Ӷ������������̼����������3.3g��
С��ͬѧ��Ϊ�÷����ⶨ�Ķ�����̼��������ƫС�������Ƿ�Ӧ�����Ķ�����̼�кܶ���������ƿ�У�û����ɢ�������У�
�ʴ�Ϊ����1��1.5g��
��2��ˮ�������Ȼ��������ӷ��������У���Ӧ�����Ķ�����̼�кܶ���������ƿ�У�û����ɢ�������У�
��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.3g
| 100 |
| x |
| 44 |
| 3.3g |
x=7.5g
ÿƬ��Ƭ�к�̼��Ƶ�����Ϊ��7.5g��5=1.5g
��2��С˳ͬѧ��Ϊ�÷����ⶨ�Ķ�����̼��������ƫ��������ˮ�������Ȼ��������ӷ��������У���ɷ�Ӧ�����ʵ�������С��402.0g���Ӷ������������̼����������3.3g��
С��ͬѧ��Ϊ�÷����ⶨ�Ķ�����̼��������ƫС�������Ƿ�Ӧ�����Ķ�����̼�кܶ���������ƿ�У�û����ɢ�������У�
�ʴ�Ϊ����1��1.5g��
��2��ˮ�������Ȼ��������ӷ��������У���Ӧ�����Ķ�����̼�кܶ���������ƿ�У�û����ɢ�������У�
������������������л�ѧ����ʽ�ļ����DZȽϳ��õķ���

��ϰ��ϵ�д�
 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ
���������У����ڴ�������ǣ�������
| A��ʯ��ʯ | B������� |
| C�������� | D����Ȫˮ |

 ���ĸ������α�ʾA��̼������Һ����B����������Һ����C���Ȼ�����Һ����D�����ۣ��������ʣ������ڵ��������й�ͬ�ı߱�ʾ�������ʿ��Է�����Ӧ����ش��������⣺
���ĸ������α�ʾA��̼������Һ����B����������Һ����C���Ȼ�����Һ����D�����ۣ��������ʣ������ڵ��������й�ͬ�ı߱�ʾ�������ʿ��Է�����Ӧ����ش��������⣺ ���������쳦��ȫ������������һ�������ʷ�����Ѿ����뵽���������з�չ��ѪҺ�У���Ϊ��������ɷָ��һ���֣����к쳡���ṩ��Ӫ���ض����������Ҫ�����ǣ�1��
���������쳦��ȫ������������һ�������ʷ�����Ѿ����뵽���������з�չ��ѪҺ�У���Ϊ��������ɷָ��һ���֣����к쳡���ṩ��Ӫ���ض����������Ҫ�����ǣ�1��