��Ŀ����
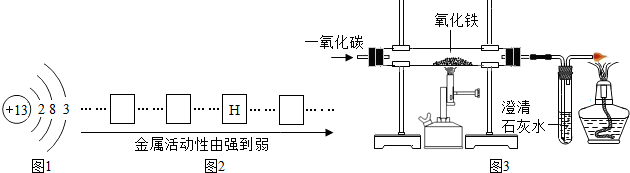
��ʽ̼��ͭ�ijɷ��ж��֣��仯ѧʽһ��ɱ�ʾΪxCu��OH��2?yCuCO3��ʵ��С��Ϊ�ⶨij��ʽ̼��ͭ��Ʒ����ɣ�������ͼ��ʾ��װ�ã��г�����ʡ�ԣ�����ʵ�飮
����1�����װ�õ������ԣ�����Ʒƽ������ƽֱ�������У�
����2������K�����������һ��ʱ���رգ��������װ�õ�������
����3������װ��Bֱ��װ��C�������ݲ������۲쵽��Ʒȫ��ת��Ϊ��ɫ��ĩ��
����4��
����5���������װ�õ�������
��1��װ��A�������� ������װ��E����ʵ��ⶨ��x/y��ֵ�� ����ƫ��ƫС����Ӱ�죩��
��2��ijͬѧ��ʵ������вɼ����������ݣ�
A����Ӧǰ����������Ʒ������163.8g B����Ӧ����������Ʒ������Ϊ56.0g
C��װ��Cʵ�������9.0g D��װ��Dʵ�������8.8g
Ϊ�ⶨ
 ��ֵ������Ϊ����ѡ���������ɼ������е� ��д��������ϵ���ĸ���ţ�һ�鼴���Խ��м��㣮���ݳ����Ľ����д������Ʒ��ɵĻ�ѧʽ ��
��ֵ������Ϊ����ѡ���������ɼ������е� ��д��������ϵ���ĸ���ţ�һ�鼴���Խ��м��㣮���ݳ����Ľ����д������Ʒ��ɵĻ�ѧʽ ��
���𰸡���������1������ʵ��Ŀ�ĺͼ�ʯ�ҵ����÷���
��2�����ݻ�ѧ����ʽ�ҳ���x��y�йص���������
����⣺��1��װ��A��ʯ���Ǹ�����������տ����е�ˮ�֣�ͬʱ�������տ����еĶ�����̼����ֹ��װ��CD���ص��������Ӱ�죬װ��E�������Ƿ�ֹ�����еĶ�����̼��ˮ��������Dװ�ã�Dװ�����������ɵĶ�����̼�ģ�Cװ�����������ɵ�ˮ�ģ����û��װ��E����ʹDװ����������ƫ�����ɵĶ�����̼����ƫ����x/y��ֵ��ƫС
��2����ʽ̼��ͭ�ֽ�Ļ�ѧ����xCu��OH��2?yCuCO3 ��x+y��CuO+xH2O+yCO2��Ҫ�ⶨ
��x+y��CuO+xH2O+yCO2��Ҫ�ⶨ ��ֵ�����Բⶨ���ɵ�ˮ�Ͷ�����̼�����������ⶨCװ�����ص�������Dװ�����ص���������ѡCD
��ֵ�����Բⶨ���ɵ�ˮ�Ͷ�����̼�����������ⶨCװ�����ص�������Dװ�����ص���������ѡCD
xCu��OH��2?yCuCO3 ��x+y��CuO+xH2O+yCO2��
��x+y��CuO+xH2O+yCO2��
18x 44y
9g 8.8g

 =
=
�������Ӧ����������Ʒ�������Լ�װ��C���ص�����������֪����ʽ������ͭ��ˮ��������ϵ������� ��ֵ���ʿ�ѡBC
��ֵ���ʿ�ѡBC
xCu��OH��2?yCuCO3 ��x+y��CuO+xH2O+yCO2��
��x+y��CuO+xH2O+yCO2��
��x+y��×80 18x
56g 9g
 =
=
 =
=
ͬ������֪��Ӧ����������Ʒ�������Լ�װ��C���ص�����������֪����ʽ������ͭ�Ͷ�����̼��������ϵ������� ��ֵ���ʿ�ѡBD
��ֵ���ʿ�ѡBD
xCu��OH��2?yCuCO3 ��x+y��CuO+xH2O+yCO2��
��x+y��CuO+xH2O+yCO2��
��x+y��×80 44y
56g 8.8g
 =
=
 =
=
�ʴ�Ϊ������4������K����������������Թ���ȴ��ر�
��1����ȥ�����е�CO2��ˮ������ƫС
��2��BC��BD��CD
��3��5Cu��OH��2?2CuCO3
���������⿼����ݻ�ѧ����ʽ�������������ɣ�Ũ�������ص����������ɵ�ˮ����������ʯ�����ص����������ɵĶ�����̼��������
��2�����ݻ�ѧ����ʽ�ҳ���x��y�йص���������
����⣺��1��װ��A��ʯ���Ǹ�����������տ����е�ˮ�֣�ͬʱ�������տ����еĶ�����̼����ֹ��װ��CD���ص��������Ӱ�죬װ��E�������Ƿ�ֹ�����еĶ�����̼��ˮ��������Dװ�ã�Dװ�����������ɵĶ�����̼�ģ�Cװ�����������ɵ�ˮ�ģ����û��װ��E����ʹDװ����������ƫ�����ɵĶ�����̼����ƫ����x/y��ֵ��ƫС
��2����ʽ̼��ͭ�ֽ�Ļ�ѧ����xCu��OH��2?yCuCO3
 ��x+y��CuO+xH2O+yCO2��Ҫ�ⶨ
��x+y��CuO+xH2O+yCO2��Ҫ�ⶨ ��ֵ�����Բⶨ���ɵ�ˮ�Ͷ�����̼�����������ⶨCװ�����ص�������Dװ�����ص���������ѡCD
��ֵ�����Բⶨ���ɵ�ˮ�Ͷ�����̼�����������ⶨCװ�����ص�������Dװ�����ص���������ѡCDxCu��OH��2?yCuCO3
 ��x+y��CuO+xH2O+yCO2��
��x+y��CuO+xH2O+yCO2��18x 44y
9g 8.8g

 =
=
�������Ӧ����������Ʒ�������Լ�װ��C���ص�����������֪����ʽ������ͭ��ˮ��������ϵ�������
 ��ֵ���ʿ�ѡBC
��ֵ���ʿ�ѡBCxCu��OH��2?yCuCO3
 ��x+y��CuO+xH2O+yCO2��
��x+y��CuO+xH2O+yCO2����x+y��×80 18x
56g 9g
 =
=
 =
=
ͬ������֪��Ӧ����������Ʒ�������Լ�װ��C���ص�����������֪����ʽ������ͭ�Ͷ�����̼��������ϵ�������
 ��ֵ���ʿ�ѡBD
��ֵ���ʿ�ѡBDxCu��OH��2?yCuCO3
 ��x+y��CuO+xH2O+yCO2��
��x+y��CuO+xH2O+yCO2����x+y��×80 44y
56g 8.8g
 =
=
 =
=
�ʴ�Ϊ������4������K����������������Թ���ȴ��ر�
��1����ȥ�����е�CO2��ˮ������ƫС
��2��BC��BD��CD
��3��5Cu��OH��2?2CuCO3
���������⿼����ݻ�ѧ����ʽ�������������ɣ�Ũ�������ص����������ɵ�ˮ����������ʯ�����ص����������ɵĶ�����̼��������

��ϰ��ϵ�д�
�����Ŀ