��Ŀ����
������ѧ֪ʶ�ش��������⣺
��1������ȼ�ϡ��Ҵ����͡������ӵ��Ҵ�����
��2��̼����泥�NH4HCO3����һ�ֻ�ѧ���ϣ���ũ�������Ľϴ���Ӫ��Ԫ�ؿ���������
A������ B��������� C�������
ʩ�û��ʿ��������ʳ������Ҳ���ܸ������ͻ���������Ⱦ�����ʩ�û��ʵĽ����ǣ�
��1������ȼ�ϡ��Ҵ����͡������ӵ��Ҵ�����
�л�
�л�
���л���������������������ʱҪ����𡱣���ȼ�յ������������ṩ���¶�
�¶�
��д���Ҵ���ȫȼ�յĻ�ѧ����ʽC2H5OH+3O2
2CO2+3H2O
| ||
C2H5OH+3O2
2CO2+3H2O
��
| ||
��2��̼����泥�NH4HCO3����һ�ֻ�ѧ���ϣ���ũ�������Ľϴ���Ӫ��Ԫ�ؿ���������
��
��
�ʣ� ijũ�����Լ���С�������ٻ���Ҷ�����ٶ�����̼����泥��㻹���������Ƽ����л����е�A
A
��A������ B��������� C�������
ʩ�û��ʿ��������ʳ������Ҳ���ܸ������ͻ���������Ⱦ�����ʩ�û��ʵĽ����ǣ�
����ʩ�û��ʣ��𰸺������ɣ�
����ʩ�û��ʣ��𰸺������ɣ�
����������1�����Ҵ��Ļ�ѧʽC2H5OH���ж��Ҵ��Ƿ����л������ȼ������ˮ�Ͷ�����̼��д����Ӧ�Ļ�ѧ����ʽ�������ȼ�յ������������
��2��̼����泥�NH4HCO3�����е�Ԫ�أ���һ�ֵ��ʣ�����ABCѡ��������
��3����ϻ���ʹ���д��ڵIJ��㣬��ʩ�û���������������飮
��2��̼����泥�NH4HCO3�����е�Ԫ�أ���һ�ֵ��ʣ�����ABCѡ��������
��3����ϻ���ʹ���д��ڵIJ��㣬��ʩ�û���������������飮
����⣺��1�����Ҵ��Ļ�ѧʽC2H5OH֪�����к���CԪ�أ������Ҵ����л����������ʱҪ����𡱣���ȼ�յ������������ṩ���¶��Ա�ﵽ��ȼ��--���͵��Ż�㣻�Ҵ���ȫȼ������ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪC2H5OH+3O2
2CO2+3H2O��
��2��̼����泥�NH4HCO3�����е�Ԫ�أ���һ�ֵ��ʣ�ijũ�����Լ���С�������ٻ���Ҷ�����٣���ȱ���˵��ʣ����˹���̼����泥������Թ������أ�����������ʣ�������Ǽطʣ�������ѡ��
��3������ʩ�û��ʻ����������ữ��ˮ���������ߣ�����Ҫ����ʩ�ʣ�
�ʴ�Ϊ����1���л��� �¶ȣ�C2H5OH+3O2
2CO2+3H2O��
��2������ A��
��3������ʩ�û��ʣ��𰸺������ɣ���
| ||
��2��̼����泥�NH4HCO3�����е�Ԫ�أ���һ�ֵ��ʣ�ijũ�����Լ���С�������ٻ���Ҷ�����٣���ȱ���˵��ʣ����˹���̼����泥������Թ������أ�����������ʣ�������Ǽطʣ�������ѡ��
��3������ʩ�û��ʻ����������ữ��ˮ���������ߣ�����Ҫ����ʩ�ʣ�
�ʴ�Ϊ����1���л��� �¶ȣ�C2H5OH+3O2
| ||
��2������ A��
��3������ʩ�û��ʣ��𰸺������ɣ���
������������Ҫ�����л�����ж�����ѧ����ʽ����д�����ʵ�ʹ�õȣ�����֪ʶ��϶࣬Ҫ����������

��ϰ��ϵ�д�
�����Ŀ
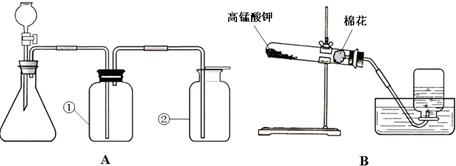
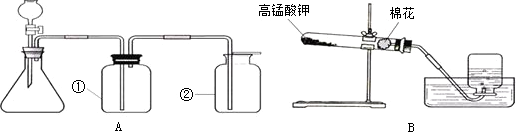
ͼA��B��ʵ���ҳ�������ȡ�����װ�ã�������ѧ֪ʶ�ش��������⣺
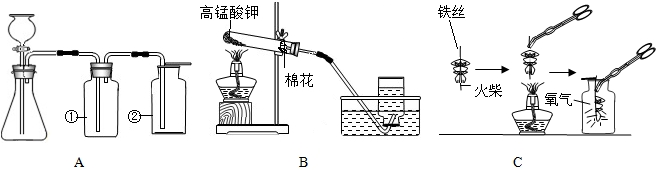
��1��װ��A�Тڵ����������� ��������װ��A����ȡ��������ʹ�õ�ҩƷ�� ���ѧʽ����������ȡCO2��Ӧѡ��װ�� ���A����B������
��2��װ��B��ʾ�����ø��������ȡ���������л�ȱ�ٵ������� ���÷�Ӧ�Ļ�ѧ����ʽΪ ��ʵ����ϣ�Ϊ��ֹˮ������Ӧ ��
��3��װ��A�����Եļ��飺�ȹرշ�Һ©�������� ������ë����סƿ�٣��� �����װ��©����
��4��ͼC������״��˿��ĩ��ϵһ������������ ��
��5��С������˿��������ȼ��Ϊʲô������������̽�����±���������þ���Ͳ�ͬ��̼������˿��þ������˿ֱ����Ϊ0.4mm������������ȼ��ʱ��ʵ������ļ�¼����������ش�
С����ͼCʵ��ʱ���ϱ��С�δ���ʵ�������� _
��˿��������ȼ��Ϊʲô��������䣿ԭ�� ��

��1��װ��A�Тڵ�����������
��2��װ��B��ʾ�����ø��������ȡ���������л�ȱ�ٵ�������
��3��װ��A�����Եļ��飺�ȹرշ�Һ©��������
��4��ͼC������״��˿��ĩ��ϵһ������������
��5��С������˿��������ȼ��Ϊʲô������������̽�����±���������þ���Ͳ�ͬ��̼������˿��þ������˿ֱ����Ϊ0.4mm������������ȼ��ʱ��ʵ������ļ�¼����������ش�
| ���� | þ�� | ��̼0.05%����˿ | ��̼0.2%����˿ | ��̼0.6%����˿ |
| ȼ��ʱ ������ |
����ȼ�գ����� ҫ�۰⣬���� |
����ȼ�� ���ٻ��� |
����ȼ�� �������� |
��δ� |
��˿��������ȼ��Ϊʲô��������䣿ԭ��