��Ŀ����
����Ŀ��ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ�����̽����
���������裩�����������ʷ�Ӧ�Ļ�ѧ����ʽΪ______�ɴ��Ʋ����Һ��һ����NaCl��������Na2CO3�����ᣮ
��ʵ��̽����
��һ����ȷ����Һ���Ƿ�������
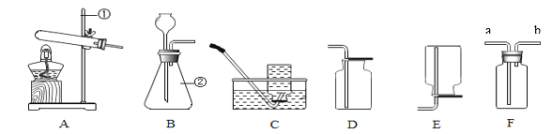
��1��ѡ���Լ�����������Ļ�ѧ���ʣ�ͬѧ��ѡ������ͼ��ʾ���������ʣ���������X�����ָʾ���е�_____��Һ��

��2��ʵ����֤��ijͬѧ���Һ�м���������þ�ۣ��۲쵽_____��ȷ����Һ��һ��û�����ᣮ
������ȷ����Һ���Ƿ���Na2CO3
��1��ijͬѧѡ��_____�����Һ��pH=10��ȷ����Һ��һ������Na2CO3
������������Һ����������
���ӷ�Һ�еõ�������NaCl�����������ʵ�鷽��������Ϊ���е���_____����ѡ����ţ�
A.����������ϡ���ᣬ�����ᾧ
B.����������Ca(OH)2��Һ�����ˡ������ᾧ
C.����������Ȼ�����Һ�����ˡ������ᾧ
���𰸡�Na2CO3+2HCl=2NaCl+CO2��+H2O ��ɫ��ʯ�� �����ݲ��������������� pH��ֽ A
��������
[��������]̼���������ᷴӦ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+CO2��+H2O�����Na2CO3+2HCl=2NaCl+CO2��+H2O��
[ʵ��̽��]
��һ��
��1��������Ļ�ѧ���ʣ���������ָʾ���е���ɫʯ����Һ��Ӧ��ʹ��ɫʯ����Һ��ɺ�ɫ�������ɫ��ʯ�
��2������������ǰ�Ľ���þ��Ӧ��������������ʵ��Ľ�����û�����ᣬ���Լ���þ�ۺ�Ӧ�����ݲ�������������ݲ���������������
������
��1������һ���Լ������Һ��pH=10���ʴ��Լ�ͨ����pH��ֽ�����pH��ֽ��
������
A������������ϡ���ᣬ�����ᾧ�ɽ�������̼���Ƴ�ȥ���õ�������NaCl���ʷ������⣻
B������������Ca(OH)2��Һ�����ˡ������ᾧ�ɽ�������̼���Ƴ�ȥ����ȴ�������µ������������ƣ��ʲ��������⣻
C������������Ȼ�����Һ�����ˡ������ᾧ�ɽ�������̼���Ƴ�ȥ�����������Ȼ��ƽ�����µ����ʣ��ʲ��������⡣���A��

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�