��Ŀ����
����Ŀ�������������Դ��������������������ȼ���Ŀ��ɡ������Ŀ��������ö�Խ��Խ�ܵ����ǵĹ�ע���ҹ��ڿ�ȼ�����Կ��ɷ�����ȡ���ش�ͻ�ƣ��γ��˹������ȵ������Բɹ��ա�
��1����ȼ����Ҫ������ˮ������飨CH4����ȫȼ�յĻ�ѧ����ʽΪ_____��
��2���������������棬�ɽ���ת��Ϊ��̬�⻯����⻯�Ƶȣ����⻯�ƣ�NaH����ˮ��Ӧ�����������ƺ��������÷�Ӧ�Ļ�ѧ����ʽΪ_____��
���𰸡�CH4+2O2![]() CO2+2H2O NaH+H2O��NaOH+H2��
CO2+2H2O NaH+H2O��NaOH+H2��
��������
����ȼ�ռ������������ڵ�ȼ�����·�Ӧ���ɶ�����̼��ˮ����ѧ����ʽ����дע����ѭ�����غ㶨�ɣ�Ҫ��ƽ����Ҫ���DZ�ע������š�
�⣺��1�������ڵ�ȼ������ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ ��
��
��2���⻯�ƣ�NaH����ˮ��Ӧ�����������ƺ��������÷�Ӧ�Ļ�ѧ����ʽΪ![]() ��
��

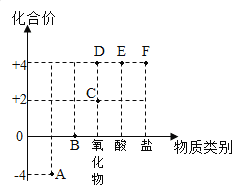
����Ŀ���±���![]() ��
��![]() �ڲ�ͬ�¶�ʱ���ܽ�ȡ�
�ڲ�ͬ�¶�ʱ���ܽ�ȡ�
�¶�/�� | 0 | 20 | 40 | 60 | 80 | |
�ܽ��/g |
| 35.7 | 36.0 | 36.6 | 37.3 | 38.4 |
| 13.3 | 31.6 | 63.9 | 110 | 169 | |
��1��50��ʱ��![]() ��ˮ�е��ܽ��_____
��ˮ�е��ܽ��_____![]() ��ˮ�е��ܽ�ȣ��С�ڡ������ڡ����ڡ�����
��ˮ�е��ܽ�ȣ��С�ڡ������ڡ����ڡ�����
��2�������ϱ�����������ϵ�л��Ƴ�![]() ��
��![]() ���ܽ�����ߣ��������ߵĽ����Ӧ���¶ȷ�Χ��_____������ţ���
���ܽ�����ߣ��������ߵĽ����Ӧ���¶ȷ�Χ��_____������ţ���
A��0�桫20�� B��20�桫40��
C��40�桫60�� D��60�桫80��
��3��ij��ȤС����ʢ��![]() ˮ���ձ�������������ͼʵ�顣
ˮ���ձ�������������ͼʵ�顣

ʵ������У�������Һ��������������һ����ȵ���span>_____������ţ�����ҺD��������_____![]() .������Һ
.������Һ![]() ��ȴ��
��ȴ��![]() �����ˣ����ɻ��յõ�_____
�����ˣ����ɻ��յõ�_____![]()
![]() ���塣����Һ�м���_____
���塣����Һ�м���_____![]() ˮϡ�ͣ��ܵõ�1����
ˮϡ�ͣ��ܵõ�1����![]() ϡ��Һ������Һ��������ʹ�á�
ϡ��Һ������Һ��������ʹ�á�
����Ŀ������ʵ������ܴﵽʵ��Ŀ�ĵ��ǣ�������
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | �Ƚ�ͭ�����Ľ������ | ��ͭƬ����Ƭ��������������Һ�� |
B | ������������ | �ֱ��ȼ�ӵ��ܷų������壬�ڻ����Ϸ�����һ���ڱ�Ϳ�г���ʯ��ˮ���ձ� |
C | �ⶨ | �ò�����պȡ��Һ�ε�ʪ���pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ������ |
D | ��ȥ | ��������建��ͨ��װ�� |
A. AB. BC. CD. D