��Ŀ����
Ϊ��ȷ�ⶨУ�Ὠ��������ʯ����̼��Ƶ�������������Уij��ѧ��ȤС���Ƚ�ʯ�Ϸ��飬Ȼ���ȡ4�ݣ��ֱ����뵽4��ϡ�����У�ʯ���е����ʲ����뷴Ӧ��������Ӧֹͣ���������ݼ�¼���±���
| ��� ��Ŀ | 1 | 2 | 3 | 4 |
| ʯ������/g | 25.0 | 25.0 | 50.0 | 50.0 |
| ϡ��������/g | 120.0 | 150.0 | 250.0 | 100.0 |
| CO2��������/g | 8.8 | 8.8 | 18.8 | 8.8 |
��1��������һ�βⶨ�����������ԵĴ���������ݵı����______��
��2��25.0gʯ��ǡ����ȫ��Ӧʱ������ϡ�����������______g��
��3�������ʯ����̼��Ƶ�����������
�⣺��1����1�κ͵�2��ʵ��ȽϿ�֪25gʯ����ȫ��Ӧֻ������8.8g������̼�������������50gʯ����ȫ��Ӧֻ������17.6g�������ܲ���18.8g������̼�����Ե�3�����ݴ���
��2����1�κ͵�2��ʵ��ȽϿ�֪25gʯ����ȫ��Ӧֻ������8.8g������̼���ٸ��ݵ�1��ʵ��͵�4��ʵ����бȽϣ���֪50gʯ����ȫ��Ӧ����17.6g������̼�����Ե�4���ǰ���100gϡ���ᷴӦ��ȡ8.8g������̼������25.0gʯ��ǡ����ȫ��Ӧʱ������ϡ�����������100g��
��3���⣺��ʯ����CaCO3������ΪX��
��CaCO3+2HCl=CaCl2+CO2��+H2O
100 44
X 8.8g
���ݣ� ��ã�X=20.0g
��ã�X=20.0g
��CaCO3����������=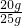 ��100%=80.0%
��100%=80.0%
��ʯ����CaCO3����������Ϊ80.0%��
�ʴ�Ϊ����1��3����2��100g����3��80.0%��
��������1�����ݵ�1�κ͵�2�αȽϼ����25gʯ����ȫ��Ӧ���ɶ�����̼���������ٸ���50gʯ�ϲ���������̼���������м��㣻��2�����ݵ�1��ʵ��͵�4��ʵ����бȽϽ��з�������3�����ݶ�����̼�����������̼��Ƶ�����������̼��Ƶ�����������Ʒ���������ɣ�
�����������ؼ��Ǹ������������ݽ��бȽϷ���������ͬ������ȡ�Ķ�����̼������ͬ���з�����
��2����1�κ͵�2��ʵ��ȽϿ�֪25gʯ����ȫ��Ӧֻ������8.8g������̼���ٸ��ݵ�1��ʵ��͵�4��ʵ����бȽϣ���֪50gʯ����ȫ��Ӧ����17.6g������̼�����Ե�4���ǰ���100gϡ���ᷴӦ��ȡ8.8g������̼������25.0gʯ��ǡ����ȫ��Ӧʱ������ϡ�����������100g��
��3���⣺��ʯ����CaCO3������ΪX��
��CaCO3+2HCl=CaCl2+CO2��+H2O
100 44
X 8.8g
���ݣ�
 ��ã�X=20.0g
��ã�X=20.0g ��CaCO3����������=
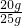 ��100%=80.0%
��100%=80.0%��ʯ����CaCO3����������Ϊ80.0%��
�ʴ�Ϊ����1��3����2��100g����3��80.0%��
��������1�����ݵ�1�κ͵�2�αȽϼ����25gʯ����ȫ��Ӧ���ɶ�����̼���������ٸ���50gʯ�ϲ���������̼���������м��㣻��2�����ݵ�1��ʵ��͵�4��ʵ����бȽϽ��з�������3�����ݶ�����̼�����������̼��Ƶ�����������̼��Ƶ�����������Ʒ���������ɣ�
�����������ؼ��Ǹ������������ݽ��бȽϷ���������ͬ������ȡ�Ķ�����̼������ͬ���з�����

��ϰ��ϵ�д�
�����Ŀ
Ϊ��ȷ�ⶨУ�Ὠ��������ʯ����̼��Ƶ�������������Уij��ѧ��ȤС���Ƚ�ʯ�Ϸ��飬Ȼ���ȡ4�ݣ��ֱ����뵽4��ϡ�����У�ʯ���е����ʲ����뷴Ӧ��������Ӧֹͣ���������ݼ�¼���±���
�����������ݣ��ش��������⣺
��1��������һ�βⶨ�����������ԵĴ���������ݵı����______��
��2��25.0gʯ��ǡ����ȫ��Ӧʱ������ϡ�����������______g��
��3�������ʯ����̼��Ƶ�����������
| ��� ��Ŀ | 1 | 2 | 3 | 4 |
| ʯ������/g | 25.0 | 25.0 | 50.0 | 50.0 |
| ϡ��������/g | 120.0 | 150.0 | 250.0 | 100.0 |
| CO2��������/g | 8.8 | 8.8 | 18.8 | 8.8 |
��1��������һ�βⶨ�����������ԵĴ���������ݵı����______��
��2��25.0gʯ��ǡ����ȫ��Ӧʱ������ϡ�����������______g��
��3�������ʯ����̼��Ƶ�����������