��Ŀ����
27�������д����л�ѧ��
��1������ݢ١��۵����⣬ѡ��ǡ������ĸ��գ�
�����Ȳ���ʱ��õ�
���˹�����ʱ��ʹ��
�۳�Ϊ����ҵ��ѪҺ���Ļ�ʯȼ����
A��������B��ʯ�ͣ�C���ɱ�
��2��Ϊ��ȷ�����彡������Ҫ��Щ������������Ӫ���ɷַḻ�����ʴ����������е���Ҫ�ɷּ��±���
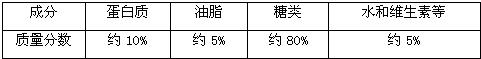
���ϱ����г������������Ӫ������
�����׳��ӹ��ɵõ����ۣ���������
��3��������ΪԤ����������Ӧ�ʵ�����
��1������ݢ١��۵����⣬ѡ��ǡ������ĸ��գ�
�����Ȳ���ʱ��õ�
A
�����˹�����ʱ��ʹ��
C
���۳�Ϊ����ҵ��ѪҺ���Ļ�ʯȼ����
B
��A��������B��ʯ�ͣ�C���ɱ�
��2��Ϊ��ȷ�����彡������Ҫ��Щ������������Ӫ���ɷַḻ�����ʴ����������е���Ҫ�ɷּ��±���

���ϱ����г������������Ӫ������
��
�࣮�����׳��ӹ��ɵõ����ۣ���������
����
������ࡱ�����ʡ�������3��������ΪԤ����������Ӧ�ʵ�����
��
Ԫ�أ���������1���ٸ�����������;�ش�
�ڸ��ݸɱ��������ȵ����ʻش�
�۸���ʯ�͵����ƻش�
��2���ٸ�������������Ӫ���ص�����ͱ�����Ϣ�ش�
�ڸ����ǵķ���ش�
��3�����ݸƵ��������ܻش�
�ڸ��ݸɱ��������ȵ����ʻش�
�۸���ʯ�͵����ƻش�
��2���ٸ�������������Ӫ���ص�����ͱ�����Ϣ�ش�
�ڸ����ǵķ���ش�
��3�����ݸƵ��������ܻش�
����⣺��1���������ܹ������������������Ȳ��ˣ�
�ڸɱ�����ʱ�����մ������ȣ�ʹ��Χ�����е�ˮ���������СҺ�Σ��γ���ˮ��
��ʯ������Ҫ�Ĺ�ҵԭ�Ϻ���Դ��������ΪҺ̬����ʯ�͵�����Ϊ����ҵ��ѪҺ����
��2���������ڵ�����Ӫ���ذ������ࡢ��֬�������ʡ�ά���ء�ˮ�����Σ�����ֻ�����Σ�
�������Ϊ���ǡ����ǡ����ǣ������Ƕ��ǣ�
��3������Ҫ�����ڹǸ�������У�ʹ�Ǻ����ݾ��м�Ӳ�Ľṹ֧�ܣ�ȱ������������Ỽ���Ͳ��������˻Ỽ�������ɣ�
�ʴ�Ϊ����1����A����C����B����2�����壻�����ࣻ��3���ƣ�
�ڸɱ�����ʱ�����մ������ȣ�ʹ��Χ�����е�ˮ���������СҺ�Σ��γ���ˮ��
��ʯ������Ҫ�Ĺ�ҵԭ�Ϻ���Դ��������ΪҺ̬����ʯ�͵�����Ϊ����ҵ��ѪҺ����
��2���������ڵ�����Ӫ���ذ������ࡢ��֬�������ʡ�ά���ء�ˮ�����Σ�����ֻ�����Σ�
�������Ϊ���ǡ����ǡ����ǣ������Ƕ��ǣ�
��3������Ҫ�����ڹǸ�������У�ʹ�Ǻ����ݾ��м�Ӳ�Ľṹ֧�ܣ�ȱ������������Ỽ���Ͳ��������˻Ỽ�������ɣ�
�ʴ�Ϊ����1����A����C����B����2�����壻�����ࣻ��3���ƣ�
��������ѧ��Դ�����������Ҳ�����������������������������صĻ�ѧ֪ʶ���غ����ǵ����桢���������ķ�չ�����п��ȵ�֮һ��

��ϰ��ϵ�д�
�����Ŀ