��Ŀ����
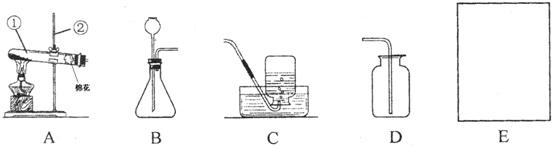
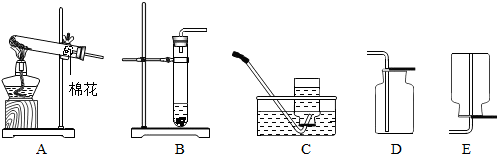
��������ʵ��װ��ͼ���ش����⣺
A B C D E
��1��д��������ŵ��������ƣ���_____________����____________��
��2����ʵ�������ù���������Һ�Ͷ������̻����ȡ����ʱ�����ж���������______���ã�������Ӧ�Ļ�ѧ����ʽΪ_____________________________________________��
��3��ʵ������ȡ������̼���壬Ӧѡ��ķ���װ��Ϊ_________����дװ�õ���ĸ���ţ���ͬ����Ӧѡ�õ��ռ�װ����___________�����������̼�Ƿ��ռ����ķ����ǣ���ȼ�ŵ�ľ������__________________���۲�����Ƿ�Ϩ��
��1���Թ� ����©��
��2���� 2H2O2 2H2O + O2��
2H2O + O2��
��3��B E ����ƿ�ڽ���:
��
��2���� 2H2O2
 2H2O + O2��
2H2O + O2����3��B E ����ƿ�ڽ���:
��

��ϰ��ϵ�д�
 ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
�����Ŀ