��Ŀ����
�о���ѧϰС���ͬѧΪ̽������������������������������ͼ��ʾװ�ã�����U������������ϱ��п̶ȣ�����˿ͨ����ܷ��ȣ��Ҳಣ���ܳ��ڣ������ͼʾʵ��ش��������⣺
��1���պϵ�Դ���أ��ɹ۲쵽����______��������������Ӧ�Ļ�ѧ����ʽΪ______��
��2��������ʵ���У��������ʲ��ܴ��������______�����ţ�
��ľ̿ ������ ��þ�� ��ͭ˿
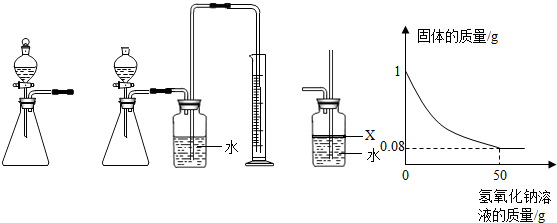
��3��ʵ������������Ͽ��Թ۲쵽U�������Һ��______����ʵ�ʹ۲쵽���Һ���ڲ�����1����λ�ô�����ʵ����������ֵ______���ƫ��ƫС���������ܵ�ԭ����______��2�㣩��
��4����ʵ�����֪��Ӧ��ʣ�����������������______��______��
��5��ͨ�����ʵ��ó��Ľ���______��
��1���պϵ�Դ���أ��ɹ۲쵽����______��������������Ӧ�Ļ�ѧ����ʽΪ______��
��2��������ʵ���У��������ʲ��ܴ��������______�����ţ�
��ľ̿ ������ ��þ�� ��ͭ˿
��3��ʵ������������Ͽ��Թ۲쵽U�������Һ��______����ʵ�ʹ۲쵽���Һ���ڲ�����1����λ�ô�����ʵ����������ֵ______���ƫ��ƫС���������ܵ�ԭ����______��2�㣩��
��4����ʵ�����֪��Ӧ��ʣ�����������������______��______��
��5��ͨ�����ʵ��ó��Ľ���______��

��1���պϵ�Դ���أ��ɹ۲쵽����ȼ�գ����������İ��̣�
�÷�Ӧ�����������ڵ�ȼ�����·�Ӧ�������������ף�
�÷�Ӧ�Ļ�ѧ����ʽΪ��4P+5O2
2P2O5���ʴ�Ϊ��ȼ�շų��������̣� 4P+5O2
2P2O5
��2����Ϊľ̿ȼ�ղ������壬�����ڿ����в���ȼ�գ�þ������������Ӧ��Ҳ�������̼��Ӧ�����Բ��ܴ�����ף�ͭ������������Ӧ���ɺ�ɫ������ͭ���壬�ʴ�Ϊ���٢ڢ�
��3�����ڿ���������Լռ���������
��װ����ȴ������ʱ���ɹ۲쵽U�������Һ��������1����ʵ�ʹ۲쵽���Һ���ڲ�����1����λ�ô�����ʵ����������ֵƫС�����ܵ�ԭ���ǣ�װ��©�������ײ��㣬�ʴ�Ϊ��������1����ƫС��װ��©�������ײ���
��4����ʵ�鲻����֪��Ӧ��ʣ����������������ǣ��������Ӧ��������ˮ���ʴ�Ϊ���������Ӧ��������ˮ
��5��ͨ�����ʵ��ó��Ľ���������Լռ�������������
���ʴ�Ϊ���������������������Ϊ
�÷�Ӧ�����������ڵ�ȼ�����·�Ӧ�������������ף�
�÷�Ӧ�Ļ�ѧ����ʽΪ��4P+5O2
| ||
| ||
��2����Ϊľ̿ȼ�ղ������壬�����ڿ����в���ȼ�գ�þ������������Ӧ��Ҳ�������̼��Ӧ�����Բ��ܴ�����ף�ͭ������������Ӧ���ɺ�ɫ������ͭ���壬�ʴ�Ϊ���٢ڢ�
��3�����ڿ���������Լռ���������
| 1 |
| 5 |
��4����ʵ�鲻����֪��Ӧ��ʣ����������������ǣ��������Ӧ��������ˮ���ʴ�Ϊ���������Ӧ��������ˮ
��5��ͨ�����ʵ��ó��Ľ���������Լռ�������������
| 1 |
| 5 |
| 1 |
| 5 |

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
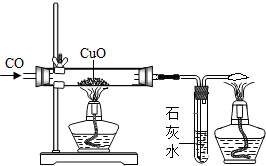 ij��ѧ�о���ѧϰС���ͬѧ��������ͼ��ʾ��̽��ʵ�飮ʵ�鷢��CO��CuO���Ⱥ��ɫ��ĩ��ɺ�ɫ��ĩ����������С����о����̲�����������
ij��ѧ�о���ѧϰС���ͬѧ��������ͼ��ʾ��̽��ʵ�飮ʵ�鷢��CO��CuO���Ⱥ��ɫ��ĩ��ɺ�ɫ��ĩ����������С����о����̲��������������о����⡿̽����ɫ��ĩ����Ҫ�ɷ�
���������ϡ�
��1���й����ʵ���ɫ��
Cu��ĩ����ɫ��CuO��ĩ����ɫ��
Cu2O��ĩ����ɫ��
��2��CuO��Cu2O���ܺ�ϡ���ᷢ����Ӧ����ѧ����ʽΪ��CuOʮH2SO4=CuSO4+H2O��Cu2O+H2SO4=CuSO4+Cu+H2O
��������ʵ�顿
��1�����Ӳ�ʲ������ں�ɫ��ĩΪһ�����ʣ���������ijɷ֣�����Ƽ�ʵ��֤����IJ²⣮
| ���� | ��ʵ�鷽�� | ���� | CO��CuO��Ӧ�Ļ�ѧ����ʽ |
A����ӦǰCuO��ĩ��������B��Ӳ�ʲ������й������ʼ��ٵ�����
C��ͨ��CO��������D����Ӧ������������������