��Ŀ����
�±�ΪԪ�����ڱ��IJ���Ԫ�ص������Ϣ�������±��ش�������⣮

��1���뻭��16��Ԫ��ԭ�ӽṹʾ��ͼ ����ѧ��Ӧ�и�ԭ�ӱȽ����� ����õ�����ʧȥ�������ӱ�����ӣ�
��2�� ��ʾ���� �������ӷ��ţ���дһ����Neԭ�ӵ��Ӳ�ṹ��ͬ�������� �������ӷ��ţ���
��ʾ���� �������ӷ��ţ���дһ����Neԭ�ӵ��Ӳ�ṹ��ͬ�������� �������ӷ��ţ���
��3���о���������ͬһ�����У������ң�ԭ��ʧȥ���ӵ������������õ����ӵ���������ǿ���ɴ��ƶϣ��ڶ������еõ���������ǿ���� ԭ�ӣ�ʧ����������ǿ���� ԭ�ӣ������ȶ��ṹ���� ԭ�ӣ���3�͵�8��Ԫ���γɻ�����Ļ�ѧʽΪ ��
��4��ijԪ�ص�ԭ�ӽṹʾ��ͼΪ ������λ��Ԫ�����ڱ��еĵ� ���ڣ�
������λ��Ԫ�����ڱ��еĵ� ���ڣ�
��5����Ԫ�غ�þԪ���γɵ��⻯þ��MgH2������Ϊ��������ṩ��Դ������Ҫԭ�����⻯þ��MgH2��������ˮ��Ӧ����������þ���������÷�Ӧ�ķ��ű���ʽΪ ��

��1���뻭��16��Ԫ��ԭ�ӽṹʾ��ͼ
��2��
 ��ʾ����
��ʾ������3���о���������ͬһ�����У������ң�ԭ��ʧȥ���ӵ������������õ����ӵ���������ǿ���ɴ��ƶϣ��ڶ������еõ���������ǿ����
��4��ijԪ�ص�ԭ�ӽṹʾ��ͼΪ
 ������λ��Ԫ�����ڱ��еĵ�
������λ��Ԫ�����ڱ��еĵ���5����Ԫ�غ�þԪ���γɵ��⻯þ��MgH2������Ϊ��������ṩ��Դ������Ҫԭ�����⻯þ��MgH2��������ˮ��Ӧ����������þ���������÷�Ӧ�ķ��ű���ʽΪ
���㣺Ԫ�����ڱ����ص㼰��Ӧ��,ԭ�ӽṹʾ��ͼ�����ӽṹʾ��ͼ,��ѧʽ����д������,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺��ѧ����������غ㶨��
��������1������ԭ�ӽṹʾ��ͼ�ĸ����ֵ�����������
��2�����ݺ˵���������������Ĺ�ϵ���жϣ�
��3��������ɵ���Ϣ�����жϣ��ȶ��ṹ��������������8�Ľṹ����һ�����Ӳ�ʱ������������2����
��4�����Ӳ���������������
��5�����ݷ�Ӧ��������Ӧ�������������غ㶨�ɿ�����д��Ӧ�Ļ�ѧ����ʽ
��2�����ݺ˵���������������Ĺ�ϵ���жϣ�
��3��������ɵ���Ϣ�����жϣ��ȶ��ṹ��������������8�Ľṹ����һ�����Ӳ�ʱ������������2����
��4�����Ӳ���������������
��5�����ݷ�Ӧ��������Ӧ�������������غ㶨�ɿ�����д��Ӧ�Ļ�ѧ����ʽ
����⣺��1������ԭ�ӽṹʾ��ͼ�Ļ���������ԭ�ӵĽṹʾ��ͼΪ ���������6�����ӣ��õ�������Ϊ8�����ȶ��ṹ�����
���������6�����ӣ��õ�������Ϊ8�����ȶ��ṹ����� ���õ���
���õ���
��2���÷��ŵĺ˵����Ϊ13��������10�����ӣ���Ϊ�����ӣ������ӡ������ӵĽṹ����ԭ�ӽṹ��ͬ�����Al3+��O2- �� F-��
��3����Ϊ��ͬ�����д����ң�ԭ��ʧȥ���ӵ������������õ����ӵ���������ǿ�����Եڶ������еõ���������ǿ����9��Ԫ��F��ʧ����������ǿ��Ӧ����3��Ԫ��Li�������ȶ��ṹ��ԭ�ӣ������һ����8�����ӣ���ֻ��һ�����Ӳ��������ӣ���ԭ�ӣ��ڶ�������Ԫ��Ne�Ǿ����ȶ��ṹ��ԭ�ӣ���3�͵�8��Ԫ�طֱ�Ϊ�Ԫ�ء���Ԫ�أ���Ͻṹʾ��ͼ����֪���ǵ������������ֱ�Ϊ1��6������Ʋ⻯�ϼ۷ֱ�Ϊ+1��-2�ۣ����γɻ�����Ļ�ѧʽΪLi2O�����F��Li�� Ne��Li2O��
��4����Ԫ�ص�ԭ�Ӻ�����5�����Ӳ㣬��λ�ڵ������ڣ�����壻
��5���⻯þ������ˮ��Ӧ�Ļ�ѧ����ʽΪ��MgH2+2H2O�TMg��OH��2+2H2����
���MgH2+2H2O�TMg��OH��2+2H2����
 ���������6�����ӣ��õ�������Ϊ8�����ȶ��ṹ�����
���������6�����ӣ��õ�������Ϊ8�����ȶ��ṹ����� ���õ���
���õ�����2���÷��ŵĺ˵����Ϊ13��������10�����ӣ���Ϊ�����ӣ������ӡ������ӵĽṹ����ԭ�ӽṹ��ͬ�����Al3+��O2- �� F-��
��3����Ϊ��ͬ�����д����ң�ԭ��ʧȥ���ӵ������������õ����ӵ���������ǿ�����Եڶ������еõ���������ǿ����9��Ԫ��F��ʧ����������ǿ��Ӧ����3��Ԫ��Li�������ȶ��ṹ��ԭ�ӣ������һ����8�����ӣ���ֻ��һ�����Ӳ��������ӣ���ԭ�ӣ��ڶ�������Ԫ��Ne�Ǿ����ȶ��ṹ��ԭ�ӣ���3�͵�8��Ԫ�طֱ�Ϊ�Ԫ�ء���Ԫ�أ���Ͻṹʾ��ͼ����֪���ǵ������������ֱ�Ϊ1��6������Ʋ⻯�ϼ۷ֱ�Ϊ+1��-2�ۣ����γɻ�����Ļ�ѧʽΪLi2O�����F��Li�� Ne��Li2O��
��4����Ԫ�ص�ԭ�Ӻ�����5�����Ӳ㣬��λ�ڵ������ڣ�����壻
��5���⻯þ������ˮ��Ӧ�Ļ�ѧ����ʽΪ��MgH2+2H2O�TMg��OH��2+2H2����
���MgH2+2H2O�TMg��OH��2+2H2����
������������Ҫ������ѧ����Ԫ�������ɺ�Ԫ�������������������Ĺ�ϵ����ʶ��Ԫ�����ڱ��У�ͬһ���ڵ�Ԫ�ص�ԭ�Ӻ�����Ӳ�����ͬ��ͬһ���Ԫ�ص�ԭ��������������ͬ�һ�ѧ���������ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
�ԡ�15%��ʳ����Һ���ĺ�����ʹ�����ǣ�������
| A��100gʳ����Һ���ܽ���15gʳ�� |
| B����ʳ�κ�ˮ����15��85�������������Һ |
| C��100gˮ���ܽ���15gʳ�� |
| D����30gʳ���ܽ���170gˮ�������Һ |
 ����������������벻������Ҫ���ϣ�
����������������벻������Ҫ���ϣ�
 �����������ij���Ԫ�أ�ÿ�ձ��������㹻���ĸƣ�Ŀǰ�г��ϵIJ���ҩ���ܶ࣬��ͼ��ij��Ʒ�ƵIJ���ҩƷ�IJ���˵���飮
�����������ij���Ԫ�أ�ÿ�ձ��������㹻���ĸƣ�Ŀǰ�г��ϵIJ���ҩ���ܶ࣬��ͼ��ij��Ʒ�ƵIJ���ҩƷ�IJ���˵���飮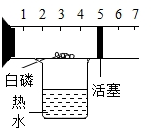 ��ͼ��һ�����п̶Ⱥͻ����ɻ����IJ��������������п����������İ��ף���������ʢ�з�ˮ���ձ��Ϸ�������ʵ�飮�����ʵ�鱨�棺
��ͼ��һ�����п̶Ⱥͻ����ɻ����IJ��������������п����������İ��ף���������ʢ�з�ˮ���ձ��Ϸ�������ʵ�飮�����ʵ�鱨�棺