��Ŀ����
Ϊ�˲ⶨ��ͭ��ͭ��п��������ɣ�ij�о���ѧϰС���ȡ��ͭ��Ʒ10g�������м���������ϡ��������β���t1��t5ʱ���������������ʵ�������¼�����ݼ��±���
��1���÷�Ӧ����������______mol�����к���______������ӣ�
��2���û�ͭ��Ʒ��п�����ʵ���Ϊ______mol��ͭ����������Ϊ______��
| ��Ӧʱ��/s | t1 | t2 | t3 | t4 | t5 |
| ����H2�������ʵ���/mol | 0.015 | 0.03 | 0.04 | 0.05 | 0.05 |
��2���û�ͭ��Ʒ��п�����ʵ���Ϊ______mol��ͭ����������Ϊ______��
��1���ɼ�¼�����ݿ�֪����Ӧ��t4��t5ʱ���������������ٸı䣬��ʱ��������Ϊ0.05mol������H2���Ӹ���=0.05��6.02��1023=3.01��1022����
��2����������п�����ʵ���Ϊx��
Zn+H2SO4�TZnSO4+H2��
1 1
x 0.05mol
=
��
x=0.05mol
�û�ͭ��Ʒ��ͭ����������=
��100%=67.5%
�ʴ�Ϊ����1��0.05��3.01��1022����2��0.05��67.5%��
��2����������п�����ʵ���Ϊx��
Zn+H2SO4�TZnSO4+H2��
1 1
x 0.05mol
| 1 |
| x |
| 1 |
| 0.05mol |
x=0.05mol
�û�ͭ��Ʒ��ͭ����������=
| 10g-0.05mol��65g/mol |
| 10g |
�ʴ�Ϊ����1��0.05��3.01��1022����2��0.05��67.5%��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
ij��ѧ��ȤС��Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ���п������������ȡ��6g�Ͻ���Ʒ����50gϡ�����5�μ�����Ʒ�У���ַ�Ӧ����ˡ�ϴ�ӡ�������أ��õ���ʵ���������£�
��1��mֵΪ ��
��2����Ͻ���п������������
��3����ϡ���������ʵ�����������
| ϡ�������� | ʣ��������� |
| ��һ�μ���10g | 4.7g |
| �ڶ��μ���10g | mg |
| �������10g | 2.1g |
| ���Ĵμ���10g | 1.2g |
| ������10g | 1.2g |
��2����Ͻ���п������������
��3����ϡ���������ʵ�����������
 Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ���9.8%��ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ�����������������Һ��������ϵ��ͼ��ʾ���Լ��㣺
Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ���9.8%��ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ�����������������Һ��������ϵ��ͼ��ʾ���Լ��㣺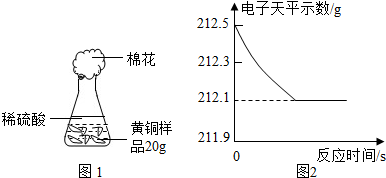
 Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ�ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ����������������������ϵ��ͼ��ʾ������ͼʾ�ش����⣺
Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ�ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ����������������������ϵ��ͼ��ʾ������ͼʾ�ش����⣺