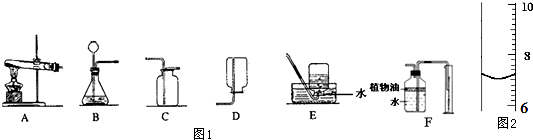
��Ŀ����
��1����Ҫ˵�����д����ʵ���������������IJ����������˵һ�ֺ�����ɣ���
�ٵι�ȡ���Լ���ƽ�Ż��� ��
���㵹ϸ��ƿ���ҩƷʱ����ǩû���������� ��
�ۼ��Ⱥ���Թܣ���������ˮ��ϴ ��
��ʵ����ʣ���ҩƷ������ˮ���� ��
�ݹ���ҩƷֱ�ӷ�����ƽ�������г��� ��
��2�������Ϸ���һ���ռ���ƿ����ͬѧ˵��������ƿ��û�ж���������ͬѧ˵������ƿ�������ʡ�������Ϊ�ĸ�ͬѧ˵�öԣ�Ϊʲô�� ��
�ٵι�ȡ���Լ���ƽ�Ż���
���㵹ϸ��ƿ���ҩƷʱ����ǩû����������
�ۼ��Ⱥ���Թܣ���������ˮ��ϴ
��ʵ����ʣ���ҩƷ������ˮ����
�ݹ���ҩƷֱ�ӷ�����ƽ�������г���
��2�������Ϸ���һ���ռ���ƿ����ͬѧ˵��������ƿ��û�ж���������ͬѧ˵������ƿ�������ʡ�������Ϊ�ĸ�ͬѧ˵�öԣ�Ϊʲô��
���㣺Һ��ҩƷ��ȡ��,������-������ƽ,����������ϴ��,�����ijɷּ����ɷֵ��������
ר�⣺������ˮ,������������ѧʵ���������
��������1����ʹ��ʱ��ͷ���ϣ��ܿ����£���ֹƽ�Ż��ã�
�ڱ�ǩ�������ģ���ֹҺ��������ʴ��ǩ��
�ۼ��Ⱥ���Թܣ���������ˮ��ϴʹ�Թ����Ȳ�����
����ʣ��ҩƷҪ����������һҪ�������Ż�ԭƿ�����涪�������ó�ʵ���ң�Ҫ����ָ��������
�ݹ���ҩƷֱ�ӷ�����ƽ�������г�������ʴ���̣�
��2������ƿ�к��п�����Ҳ�������ʣ�
�ڱ�ǩ�������ģ���ֹҺ��������ʴ��ǩ��
�ۼ��Ⱥ���Թܣ���������ˮ��ϴʹ�Թ����Ȳ�����
����ʣ��ҩƷҪ����������һҪ�������Ż�ԭƿ�����涪�������ó�ʵ���ң�Ҫ����ָ��������
�ݹ���ҩƷֱ�ӷ�����ƽ�������г�������ʴ���̣�
��2������ƿ�к��п�����Ҳ�������ʣ�
����⣻��1���ٵι�ȡ���Լ���ƽ�Ż��ã�Һ���Լ����뽺ͷ��ʹ��ͷ�ܸ�ʴ��ͷ������ʴ�����Һ��
���㵹ϸ��ƿ���ҩҺʱ����ǩû�������ģ�Һ��������ʴ��ǩ��
�ۼ��Ⱥ���Թܣ���������ˮ��ϴʹ�Թ����Ȳ�������ը���Թܣ�
��ʵ����ʣ���ҩƷ������ˮ���У�����ʴ��ˮ������Ⱦˮ�壮
�ݹ���ҩƷֱ�ӷ�����ƽ�������г�������ʴ���̣�
��2������ƿ�к��п�����Ҳ�������ʣ�
�ʴ�Ϊ����1����Һ���Լ����뽺ͷ��ʹ��ͷ�ܸ�ʴ��ͷ������ʴ�����Һ��
��Һ��������ʴ��ǩ��
��ը���Թܣ�
������ʴ��ˮ������Ⱦˮ�壻
�ݸ�ʴ���̣�
��2���ң�����ƿ�к��п�����
���㵹ϸ��ƿ���ҩҺʱ����ǩû�������ģ�Һ��������ʴ��ǩ��
�ۼ��Ⱥ���Թܣ���������ˮ��ϴʹ�Թ����Ȳ�������ը���Թܣ�
��ʵ����ʣ���ҩƷ������ˮ���У�����ʴ��ˮ������Ⱦˮ�壮
�ݹ���ҩƷֱ�ӷ�����ƽ�������г�������ʴ���̣�
��2������ƿ�к��п�����Ҳ�������ʣ�
�ʴ�Ϊ����1����Һ���Լ����뽺ͷ��ʹ��ͷ�ܸ�ʴ��ͷ������ʴ�����Һ��
��Һ��������ʴ��ǩ��
��ը���Թܣ�
������ʴ��ˮ������Ⱦˮ�壻
�ݸ�ʴ���̣�
��2���ң�����ƿ�к��п�����
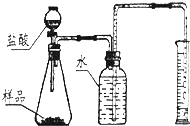
������������Ҫ����һЩʵ���ע������˽�ʵ����ҩƷ��ȡ�÷�����ԭ���˽�ҩƷ��ȡ�õĺ�ʣ��ҩƷ�ġ�������ԭ��

��ϰ��ϵ�д�
�����Ŀ
���л�ѧ����ʽ��д��ȷ������Ӧ��һ�µ��ǣ�������
| A���ú��׳�ȥ�ܱ������ڵ�������4P+5O2�T2P2O5 |
| B����ϡ��������⣺2HCl+FeO�TFeCl2+H2O |
| C���ö�����̼��̼�����ϣ�2CO2��+H2O�TH2CO3 |
| D���ó���ʯ��ˮ�������������ѱ��ʣ�Na2CO3+Ca��OH��2�TCaCO3��+2NaOH |
 ij��ȤС���һ������������Ʒ��ڱ��������ʱ�䱩¶�ڿ����У����ֹ��������ʪ�����ܻ���Һ��������γɾ��壬���ձ�ɷ�ĩ����ش��������⣺
ij��ȤС���һ������������Ʒ��ڱ��������ʱ�䱩¶�ڿ����У����ֹ��������ʪ�����ܻ���Һ��������γɾ��壬���ձ�ɷ�ĩ����ش��������⣺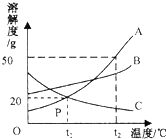 ��ͼ��A��B��C���ֹ������ʵ��ܽ�����ߣ�
��ͼ��A��B��C���ֹ������ʵ��ܽ�����ߣ�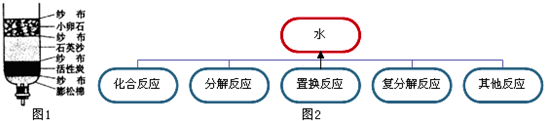

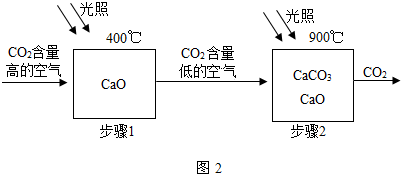
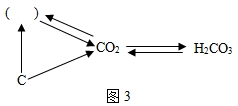
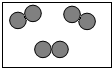 ȫ��ӵ�еĻ������ѳ���3000���֣����в������������⡢���������е�ijЩԪ����ɣ���������4��Ԫ�أ���Ҫ����Ԫ�ط��Ż�ѧʽ��գ�
ȫ��ӵ�еĻ������ѳ���3000���֣����в������������⡢���������е�ijЩԪ����ɣ���������4��Ԫ�أ���Ҫ����Ԫ�ط��Ż�ѧʽ��գ�