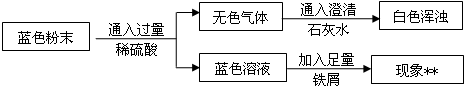
��Ŀ����
ij�о���ѧϰС��ԡ�H2S��ˮ��Һ�Ƿ������ԡ�����̽��������������ǵ�̽��������ش��й����⡣

��1���������ϣ�
��H2S��һ����ɫ���г�������ζ���ж����壬������ˮ���ڳ��³�ѹ�£�1���ˮ�����ܽ�2.6������⣻�ܶȱȿ����Ĵ�
��ʵ�����ͨ������������FeS�������ϡ�����ϡ���ᷴӦ��ȡ���⡣
��2��������裺H2S��ˮ��Һ������
��3��ʵ��̽����
���Ʊ�H2S
������ϡ�����Ʊ�H2S���仯ѧ����ʽΪ_______________��
����ȡH2S�ķ���װ��Ϊ_________������ĸ�����ռ�װ��ֻ��ѡD��ԭ����_____________��
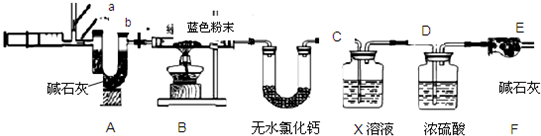
���������ͼ��ʾװ�ý�����֤ʵ�顣
��H2S��һ����ɫ���г�������ζ���ж����壬������ˮ���ڳ��³�ѹ�£�1���ˮ�����ܽ�2.6������⣻�ܶȱȿ����Ĵ�
��ʵ�����ͨ������������FeS�������ϡ�����ϡ���ᷴӦ��ȡ���⡣
��2��������裺H2S��ˮ��Һ������
��3��ʵ��̽����
���Ʊ�H2S
������ϡ�����Ʊ�H2S���仯ѧ����ʽΪ_______________��
����ȡH2S�ķ���װ��Ϊ_________������ĸ�����ռ�װ��ֻ��ѡD��ԭ����_____________��
���������ͼ��ʾװ�ý�����֤ʵ�顣

��ʵ������У�Aװ������ɫʯ����ֽ����ɫʼ��û�б仯��Aװ�õ�������______________��
����ͨ��H2S֮ǰ��Bװ���н�ͷ�ι��ڵ�����ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯���˲�����Ŀ����_______________������H2Sͨ��ʱ����ʪ�����ɫʯ����ֽ��죬������˵��_____________��
��4�����ۣ�ԭ���������
��5����˼�����ۣ�����֤ʵ���У���һ��������©������ָ����©֮��Ϊ_______________��
��6��֪ʶ��չ��H2S��ˮ��Һ�����ԣ��������ͨ�ԣ���������������Һ����β������Ӧ�Ļ�ѧ����ʽΪ_________________��
����ͨ��H2S֮ǰ��Bװ���н�ͷ�ι��ڵ�����ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯���˲�����Ŀ����_______________������H2Sͨ��ʱ����ʪ�����ɫʯ����ֽ��죬������˵��_____________��
��4�����ۣ�ԭ���������
��5����˼�����ۣ�����֤ʵ���У���һ��������©������ָ����©֮��Ϊ_______________��
��6��֪ʶ��չ��H2S��ˮ��Һ�����ԣ��������ͨ�ԣ���������������Һ����β������Ӧ�Ļ�ѧ����ʽΪ_________________��
��FeS+2HCl==FeCl2+H2S��
��A��H2S����ˮ���ܶȱȿ�����
��֤��H2S���岻��ʹ��ɫʯ����ֽ��ɫ��������H2S�������ԣ�
��֤��ˮ����ʹ��ɫʯ����ֽ��ɫ����֤��ˮ�����ԣ��������ˮ��Һ������
��5��û��β�������������գ�װ��
��6��2NaOH+H2S==Na2S+2H2O
��A��H2S����ˮ���ܶȱȿ�����
��֤��H2S���岻��ʹ��ɫʯ����ֽ��ɫ��������H2S�������ԣ�
��֤��ˮ����ʹ��ɫʯ����ֽ��ɫ����֤��ˮ�����ԣ��������ˮ��Һ������
��5��û��β�������������գ�װ��
��6��2NaOH+H2S==Na2S+2H2O

��ϰ��ϵ�д�
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д�
�����Ŀ