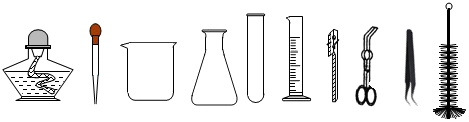
��Ŀ����
11��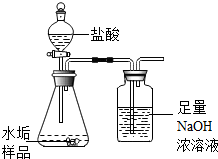 �����ձ�Ѷ��³ɽ���ŵ������������ˮ�տ�����������ˮ�����������귢����ʯ����������ϣ���йز����ܰ����ǽ����ˮ���⣮ˮ������Ҫ�ɷ���̼��ƺ�������þ������ʱ����ֻ�������ֳɷ֣�����������������Ϊ�˲ⶨˮ����������þ�ĺ�����ʵ��С��ȡˮ����Ʒ16.9g��������ͼ��ʾװ�ý���ʵ�飬ʵ�����Ҳ�װ�õ�����������4.4g����
�����ձ�Ѷ��³ɽ���ŵ������������ˮ�տ�����������ˮ�����������귢����ʯ����������ϣ���йز����ܰ����ǽ����ˮ���⣮ˮ������Ҫ�ɷ���̼��ƺ�������þ������ʱ����ֻ�������ֳɷ֣�����������������Ϊ�˲ⶨˮ����������þ�ĺ�����ʵ��С��ȡˮ����Ʒ16.9g��������ͼ��ʾװ�ý���ʵ�飬ʵ�����Ҳ�װ�õ�����������4.4g������1��ˮ����Ʒ��̼��Ƶ�������
��2����ˮ����������þ�ĺ���������������ȷ��0.1��
���� �Ҳ�װ�����������ɵĶ�����̼�ģ�����Bװ�õ�����������Ϊ������̼����������������Ʒ�е�̼��Ƶ���������������������þ������
��� �⣺���������Ҫ��ȷ����ǰ�ᣬ��һֻ�ܼ���ˮ�������ֳɷ֣������ܲ��÷Ǵ˼��˵ķ�������������þ���ڶ��Ҳ�װ�õ��������Ӽ�����Ϊ���ɶ�����̼ȫ����B���գ���������װ���Dz����ܵģ���Ϊ���ɶ�����̼�����������װ�ü�������ܽ����Ҳࣩ��
�Ҳ�װ�õ��������Ӿ������ɶ�����̼����������������̼������Ϊ4.4g��
��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
$\frac{100}{44}$=$\frac{x}{4.4g}$
x=10g��
��������þ������Ϊ16.9g-10g=6.9g��
�𣺣�1��ˮ����Ʒ��̼��Ƶ�����10g��
��2����ˮ����������þ�ĺ���Ϊ6.9g��
���� ������ʵ���ƾ���Ŀ������ʵ�����к���ʵ�֣���Ϊ�Ǵ˼��˵��ų�������������������Ϊǰ��ģ�������Ƕ�����̼������ȷ����Ӧ�ÿ������װ����ʣ�������̼���µ�Ӱ�죬������ʵ����Ҫע���һ���Ľ���

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�| A�� | ˮ����ˮ | B�� | ������������ | ||
| C�� | ������������ʯ��ʯ | D�� | ��̿��������¯β�� |
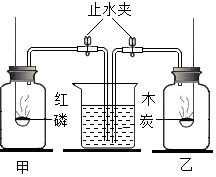 ��ͼ��ʾ��������ļ���������ƿ�ڳ���������ȼ�ճ���ֱ�ʢ�й����ĺ���ľ̿����ȼʹ���ַ�Ӧ����ȴ�����£���ֹˮ�У��۲쵽�������ǣ�������
��ͼ��ʾ��������ļ���������ƿ�ڳ���������ȼ�ճ���ֱ�ʢ�й����ĺ���ľ̿����ȼʹ���ַ�Ӧ����ȴ�����£���ֹˮ�У��۲쵽�������ǣ�������| A�� | ��ƿû��ˮ���룬��ƿ��ˮ���� | B�� | ������ƿ����ˮ���� | ||
| C�� | ��ƿ��ˮ���룬��ƿû�� | D�� | ������ƿ��û�� |
| A�� | Na2CO3--���������������� | |
| B�� | 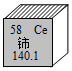 --������ԭ������Ϊ140.1g --������ԭ������Ϊ140.1g | |
| C�� | 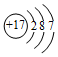 --��ԭ���ڷ�Ӧ���õ��� --��ԭ���ڷ�Ӧ���õ��� | |
| D�� | $\stackrel{+2}{Mg}$--��+2����ʾþ���Ӵ���������λ����� |
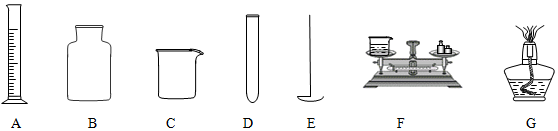
��1��д������E������ȼ����
��2������ǰ���ֲ��������������ھƾ�����ֱ�Ӽ�����DE������ţ�
��3����ȡһ�������Һ��ѡ�õ�������A�� ����ʱ����Ҫ��Һ�尼Һ�����ʹ���ˮƽ��
��4��������E��B��������������ȼ�յ�ʵ��ʱ��Ԥ����B�з�����ˮ��Ŀ���ǣ����ն�������ֹ��Ⱦ����
��5��ʵ������Dȡ��ҩƷ�����û��˵����������Ӧ��ȡ��������Һ��1-2ml����������Թܵײ����ɣ�
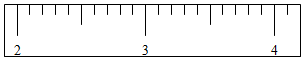
��6����ͼ����F����ij�ձ�����������������Ǹ��ձ�����Ϊ37.7g�����������������ƶ�����Ĺ��̱�ʾ��������������ʾ�������룬��������ʾȡ�����룻��ǩ�ֱ����Ͽ̶ȳ����á�|����ʾ�������ƶ�����λ�ã�
| �����С/g | 100 | 50 | 20 | 20 | 10 | 5 |
| ȡ����� | ���� |
��7����֤�ƾ���Gȼ�պ�����ˮ��ʵ��������Ƚ��ƾ��Ƶ�ȼ���ڻ����Ϸ���һ��������ձ����ձ��ڱڳ���ˮ����֤���ƾ�ȼ�պ���ˮ���ɣ�