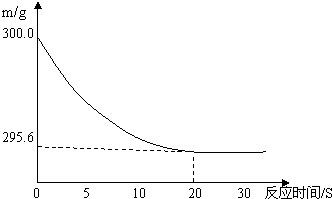
��Ŀ����
��ʢ��22.3 g Na2CO3��NaCl����������ձ��м���216.1gϡ����ǡ�÷�Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ��������m���뷴Ӧʱ�䣨t���Ĺ�ϵ����ͼ��ʾ���ձ���ͬҩƷ����ʼ����Ϊ300 g��
�ش��������⣺
��1��������������ϡ����ǡ����ȫ��Ӧʱ������ʱ��ԼΪS��
��2����ȫ��Ӧ����������̼��������g��
��3����Ӧ�õ������µIJ�������Һ��������Һ�����ʵ���������Ϊ���٣�
���𰸡���������1���ӷ�Ӧ��֮���ϵ������ͼ���Կ�������20sʱ����Ӧ���߱��ֱ�ߣ�˵����Ӧ�����������������ٱ仯��˵����ʱ������ȫ��Ӧ��
��2���ӷ�Ӧ��֮���ϵ������ͼ���Կ�������ȫ��Ӧ���ձ���ͬҩƷ��������m��Ϊ259.6g���ȷ�Ӧǰ���ٵ������������ɵ������������
��3�����ݻ�ѧ����ʽ���ó�������֮��������ȣ��г�����ʽ�����ɼ�������뷴Ӧ��̼���Ƶ������ͷ�Ӧ���ɵ��Ȼ��Ƶ�������Ȼ����ݷ�Ӧ���ɵ��Ȼ��Ƶ�����+��������Ȼ��Ƶ�����=��������Һ���������������������+ϡ��������-������������=��������Һ���������ٸ�����������������ʽ���㼴�ɣ�
����⣺��1���ӷ�Ӧ��֮���ϵ������ͼ���Կ�������20sʱ����Ӧ���߱��ֱ�ߣ�˵����Ӧ�����������������ٱ仯��˵����ʱ������ȫ��Ӧ���ʴ�Ϊ��20��
��2��300g-259.6g=4.4g���ʴ�Ϊ��4.4��
��3���⣺����뷴Ӧ��̼���Ƶ�����Ϊx����Ӧ���ɵ��Ȼ��Ƶ�����Ϊy��
Na2CO3+2HCl=2NaCl+CO2��+H2O��
106 117 44
x y 4.4g
�� ��
��
��ã�x=10.6g��y=11.7g��
�ձ��ﲻ������Һ������������Ϊ��11.7g+��22.3g-10.6g��=23.4g��
�ձ��ﲻ������Һ������Ϊ��22.3g+216.1g-4.4g=234g��
���ò�������Һ���������������� ×100%=10%��
×100%=10%��
��������Һ�����ʵ���������Ϊ10%��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м����������
��2���ӷ�Ӧ��֮���ϵ������ͼ���Կ�������ȫ��Ӧ���ձ���ͬҩƷ��������m��Ϊ259.6g���ȷ�Ӧǰ���ٵ������������ɵ������������
��3�����ݻ�ѧ����ʽ���ó�������֮��������ȣ��г�����ʽ�����ɼ�������뷴Ӧ��̼���Ƶ������ͷ�Ӧ���ɵ��Ȼ��Ƶ�������Ȼ����ݷ�Ӧ���ɵ��Ȼ��Ƶ�����+��������Ȼ��Ƶ�����=��������Һ���������������������+ϡ��������-������������=��������Һ���������ٸ�����������������ʽ���㼴�ɣ�
����⣺��1���ӷ�Ӧ��֮���ϵ������ͼ���Կ�������20sʱ����Ӧ���߱��ֱ�ߣ�˵����Ӧ�����������������ٱ仯��˵����ʱ������ȫ��Ӧ���ʴ�Ϊ��20��
��2��300g-259.6g=4.4g���ʴ�Ϊ��4.4��
��3���⣺����뷴Ӧ��̼���Ƶ�����Ϊx����Ӧ���ɵ��Ȼ��Ƶ�����Ϊy��
Na2CO3+2HCl=2NaCl+CO2��+H2O��
106 117 44
x y 4.4g
��
 ��
��
��ã�x=10.6g��y=11.7g��
�ձ��ﲻ������Һ������������Ϊ��11.7g+��22.3g-10.6g��=23.4g��
�ձ��ﲻ������Һ������Ϊ��22.3g+216.1g-4.4g=234g��
���ò�������Һ����������������
 ×100%=10%��
×100%=10%����������Һ�����ʵ���������Ϊ10%��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м����������

��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ
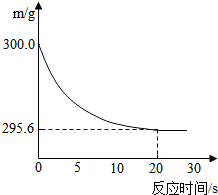 ��ʢ��22.3 g Na2CO3��NaCl����������ձ��м���216.1 gϡ����ǡ�÷�Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ��������m���뷴Ӧʱ�䣨t���Ĺ�ϵ����ͼ��ʾ���ձ���ͬҩƷ����ʼ����Ϊ300 g���ش��������⣺
��ʢ��22.3 g Na2CO3��NaCl����������ձ��м���216.1 gϡ����ǡ�÷�Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ��������m���뷴Ӧʱ�䣨t���Ĺ�ϵ����ͼ��ʾ���ձ���ͬҩƷ����ʼ����Ϊ300 g���ش��������⣺