��Ŀ����
15��ijʵ��С���ͬѧ�����õ���ͭ��ȡCu��OH��2������Ʒ�����ͼ1��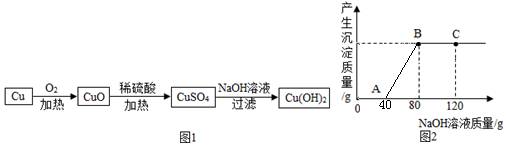
��1��ͭ������ת��ΪCuO�Ĺ����У��۲쵽�������Ǻ�ɫ�����ڣ�
��2��ȡһ������CuO����ʢ��һ������19.6%ϡ������Һ���ձ��г�ַ�Ӧ�����ձ�����μ���10%��NaOH��Һ������������������������NaOH��Һ�����Ĺ�ϵ������ͼ2��ʾ����C����A����ȣ���Һ�ж������ʣ�NaOH��
��3������Cu��OH��2�����������Ƕ��ٿˣ�
��4��B��ʱ����Һ�����ʵ������Ƕ��ٿˣ�
��5��ʵ�����õ�ϡ���������Ƕ��ٿˣ�
���� ����ͭ�Ǻ�ɫ������ͭ�Ǻ�ɫ���з�����𣻸����������������������ͭ�Ļ����Һ�ķ�Ӧ���з�����𣬸����������ṩ�������Լ���ѧ����ʽ���з�����ɣ�
��� �⣺��1��ͭ�Ǻ�ɫ�ģ���������Ӧ���ɵ�����ͭ�Ǻ�ɫ�ģ��ʻ�۲쵽��ɫ�����ɺ�ɫ���������ɫ�����ڣ�
��2������ͭ�������ᷴӦ��������ͭ��ȡһ������CuO����ʢ��һ������19.6%ϡ������Һ���ձ��г�ַ�Ӧ�����ձ�����μ���10%��NaOH��Һ������ͼ����Կ�������ʼû�г������ɣ��ʿ�ʼ������������ʣ������ᷴӦ���ﵽA��ʱ����ǡ����ȫ��Ӧ����ʱ������������ͭ�������ƣ�����C��ʱ����������ʣ�࣬��ʱ�������������ƺ�ʣ����������ƣ��ʶ���������������ƣ����NaOH��
��3������ͼʾ���Կ�����������ͭ��Ӧ������������Һ������Ϊ40g�����������Ƶ�����Ϊ��40g��10%=4g�������ɵ�������ͭ������Ϊx
2NaOH+CuSO4�TCu��OH��2��+Na2SO4
80 98
4g x
$\frac{80}{98}=\frac{4g}{x}$
x=4.9g
��4�����ݷ�Ӧ�Ļ�ѧ����ʽ��H2SO4+2NaOH�TNa2SO4+2H2O��2NaOH+CuSO4�TCu��OH��2��+Na2SO4���ʿ��Եó���2NaOH--Na2SO4��
���ĵ��������Ƶ�������Ϊ��80g��10%=8g
�����ɵ������Ƶ�����Ϊy
2NaOH--Na2SO4��
80 142
8g y
$\frac{80}{142}=\frac{8g}{y}$
y=14.2g
��5��CuO+H2SO4�TCuSO4+H2O��2NaOH+CuSO4�TCu��OH��2��+Na2SO4��H2SO4+2NaOH�TNa2SO4+2H2O�����Եó�H2SO4--2NaOH
�����������Ϊz
H2SO4--2NaOH
98 80
z 8g
$\frac{98}{80}=\frac{z}{8g}$
z=9.8g
��ϡ���������Ϊ��$\frac{9.8g}{19.6%}$=50g
�𣺣�3������Cu��OH��2������������4.9g��
��4��B��ʱ����Һ�����ʵ�������14.2g��
��5��ʵ�����õ�ϡ����������50g��
���� ���⿼����Ǹ��ݻ�ѧ����ʽ�ļ����֪ʶ����ɴ��⣬�����������е�֪ʶ���У�

 ��������ƽ�����߸���һֻ���������ձ�������ֻ�ձ��зֱ�ע����ͬ��������ͬ����������ϡ���ᣬ��ƽƽ�⣮��������ձ���Ͷ��������п�������ұ��ձ���Ͷ���������þ������ͼ��ʾ��������ַ�Ӧ����п����þ������ʣ�࣬����ƽָ�루������
��������ƽ�����߸���һֻ���������ձ�������ֻ�ձ��зֱ�ע����ͬ��������ͬ����������ϡ���ᣬ��ƽƽ�⣮��������ձ���Ͷ��������п�������ұ��ձ���Ͷ���������þ������ͼ��ʾ��������ַ�Ӧ����п����þ������ʣ�࣬����ƽָ�루������| A�� | ������ƫ��������ƫ | B�� | ������ƫ��������ƫ | ||
| C�� | һֱ����ƫ | D�� | һֱ����ƫ |
| ���� | �������� | ��ȥ���ʵķ��� | |
| A | O2 | CO | ��ȼ������� |
| B | NaOH | NaCl | ������ϡ������Һ |
| C | CuO | Cu | �ڴ��������м��� |
| D | H2 | ˮ���� | ͨ��ʢ��Ũ�����ϴ��ƿ |
| A�� | A | B�� | B | C�� | C | D�� | D |
 Ϊ�˲ⶨijʯ��ʯ��̼��Ƶ�����������������ȤС�����������ʵ�飺��ȡ10��ʯ��ʯ�����ձ�����ձ��м���������������������Ϊ10%��ϡ������Һ����Ӧ�������ձ��������������ı仯��ͼ��ʾ��
Ϊ�˲ⶨijʯ��ʯ��̼��Ƶ�����������������ȤС�����������ʵ�飺��ȡ10��ʯ��ʯ�����ձ�����ձ��м���������������������Ϊ10%��ϡ������Һ����Ӧ�������ձ��������������ı仯��ͼ��ʾ��

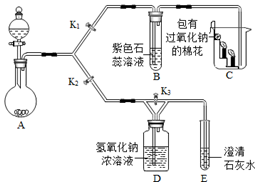
 �������Ƴ��ڴ�����ױ��ʣ�ijС����ʵ��ʱȡ��һƿ��Ŷ�������������������������ȡ12.2g��Ʒ������32.8gˮ���γ�����Һ��Ȼ���μ�29.2%�������ַ�Ӧ�������������������ƿ�����ʵ�������ϵ��ͼ��
�������Ƴ��ڴ�����ױ��ʣ�ijС����ʵ��ʱȡ��һƿ��Ŷ�������������������������ȡ12.2g��Ʒ������32.8gˮ���γ�����Һ��Ȼ���μ�29.2%�������ַ�Ӧ�������������������ƿ�����ʵ�������ϵ��ͼ��