��Ŀ����
ij�Ƽ�ʵ��С��Ϊ�˲ⶨij�������̼��Ƶĺ�������ȡ22.2g���������ۣ����������Ĵθ������գ����ʲ��μӷ�Ӧ����ѧ����ʽ��CaCO3
CaO+CO2��������ȴ��������������£�
�Լ��㣺
��1����ȫ��Ӧ������CO2��������______�ˣ�
��2�����������̼��Ƶ�����������д��������̣���������ȷ��0.1%����
| ||
| �������� | ��1�� | ��2�� | ��3�� | ��4�� |
| ʣ�����������g�� | 17 | 15.8 | 13.4 | 13.4 |
��1����ȫ��Ӧ������CO2��������______�ˣ�
��2�����������̼��Ƶ�����������д��������̣���������ȷ��0.1%����
��1�����ɶ�����̼������Ϊ��22.2g-13.4g=8.8g�����8.8��
��2��������8.8g������̼��Ҫ̼��Ƶ�����Ϊx
CaCO3
CaO+CO2��
100 44
x 8.8g
=
x=20g
̼��Ƶ���������Ϊ��
��100%��90.1%
�𣺸��������̼��Ƶ�����������90.1%��
��2��������8.8g������̼��Ҫ̼��Ƶ�����Ϊx
CaCO3
| ||
100 44
x 8.8g
| 100 |
| x |
| 44 |
| 8.8g |
̼��Ƶ���������Ϊ��
| 20g |
| 22.2g |
�𣺸��������̼��Ƶ�����������90.1%��

��ϰ��ϵ�д�
�����Ŀ
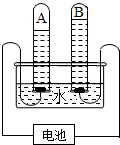
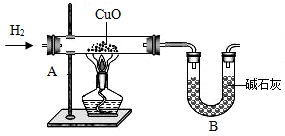


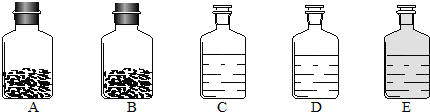
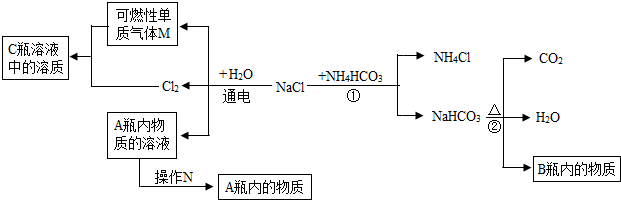
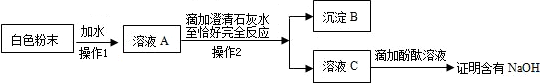
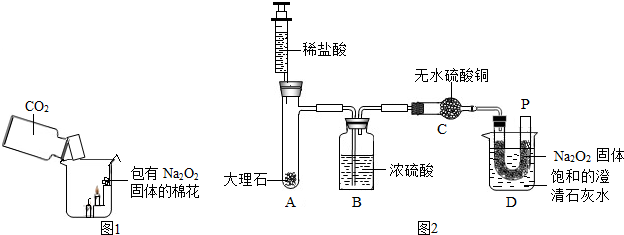
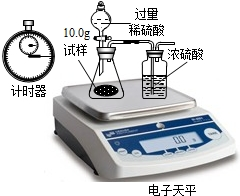
 ��2012?��������ģ��������̽��ˮ����ɵ�ʵ�飮��ͼ�ǵ��ˮʵ���ʾ��ͼ��
��2012?��������ģ��������̽��ˮ����ɵ�ʵ�飮��ͼ�ǵ��ˮʵ���ʾ��ͼ��