��Ŀ����
��5�֣�ij��ȤС��ͬѧ��ʵ�����Ʊ�������������������̽��ʵ�顣
��1��Ϊ̽�����������������طֽ��ٶȵ�Ӱ�죬��������¶Ա�ʵ�飺
��x g KClO3��1.0 g MnO2���Ȼ�ϼ���
��3.0 g KClO3��1.0 g CuO���Ȼ�ϼ�������ͬ�¶��£��Ƚ�����ʵ�����O2�Ŀ�����
����x��ֵӦΪ________�����з�Ӧ�Ļ�ѧ����ʽ��________��
��2����̽����Ӱ��˫��ˮ�ֽ��ٶȵ�ij�����ء�ʵ�����ݼ�¼���£�
| �� | ˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2����� |
| �� | 50.0 g | 1% | 0.1 g | 9 mL |
| �� | 50.0 g | 2% | 0.1 g | 16 mL |
| �� | 50.0 g | 4% | 0.1 g | 31 mL |

A������������ ��B�������������� ��C����������������D
ʵ����ۣ�����ͬ�����£�___________��������ͼװ�ý���ʵ�飬ͨ���Ƚ�__________Ҳ�ܴﵽʵ��Ŀ�ġ�

����1��3.0�������� 2KClO3 2KCl + 3O2��
2KCl + 3O2��
��2��CD�� ����ͬ�����£�˫��ˮ��Ũ��Խ��������������Խ�죨��˫��ˮ�ֽ������Խ�죩���� ͨ���Ƚ���ͬʱ���ڣ�������ƽʾ���ı仯��С��
���������������1���������Ա�ʵ��ʱҪ������ֻ��һ��������ͬ��Ҳ��������ʵ��ֻ��һ��������ͬ������������Ҫ��ͬ������ܹ��ж������ͬ�������Dz���Ӱ�����ء���͢�����ʵ���еIJ�ͬ����ѡ���˲�ͬ�Ĵ������������������Ӧ��ͬ������x��ֵӦΪ3.0�ˡ�ʵ���������ͭ�����������Ի�ѧ����ʽΪ2KClO3 2KCl + 3O2��
2KCl + 3O2��
��2����ʵ��װ�ñ��뱣֤�ų�ˮ�ĵ���Ӧ�ӽ�����ƿ�ĵײ�����ѡCDװ�ã���ʵ�����ݿ�֪����ͬ�����£�˫��ˮ��Ũ��Խ��������������Խ�졣ͨ������װ��ͼ��֪����ͼ��ͨ���Ƚϵ�λʱ���ڻ�����ͬʱ���ڵ�����ƽʾ���ı仯��С�ﵽʵ��Ŀ�ġ�
�����㣺ʵ�����Ʊ�����������̽��ʵ�顣
����������ͨ���任����̽��Ӱ��ʵ�����Ʊ��������ʵ����أ��ؼ���Ҫ���������Ա�ʵ��ʱҪ������ֻ��һ��������ͬ��Ҳ��������ʵ��ֻ��һ��������ͬ������������Ҫ��ͬ������ܹ��ж������ͬ�������Dz���Ӱ�����ء�

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
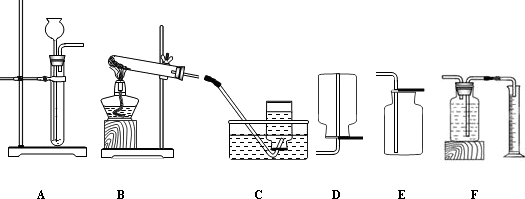

 ij��ȤС��ͬѧ��ʵ�����Ʊ������������������������ʽ�������̽����
ij��ȤС��ͬѧ��ʵ�����Ʊ������������������������ʽ�������̽����

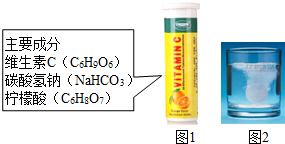 άC����Ƭ����Ҫ�ɷּ�ͼ1������ˮ�����������ݲ�������ͼ2����ij��ȤС��ͬѧ������ijɷֽ�������̽����
άC����Ƭ����Ҫ�ɷּ�ͼ1������ˮ�����������ݲ�������ͼ2����ij��ȤС��ͬѧ������ijɷֽ�������̽����