��Ŀ����
��һ�ձ���ʢ��22.3��̼���ƺ��Ȼ�����ɵĹ�������������μ�������������Ϊ10%��ϡ���ᣨNa2CO3+2HCl=2NaCl+H2O+CO2�������ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺

��1�����μ���73��ϡ����ʱ���ų������������Ϊ �� �ˡ�
��2�����μ�ϡ������ͼ�е�B��ʱ���ձ�����Һ��������ǣ�д��ѧʽ���� �� ��
��3�����μ���73��ϡ����ʱ����A��ʱ�����ձ���Ϊ��������Һ����ͨ�����������ʱ������Һ�����ʵ�������������ȷ��0.1%��
�š�4.4���ơ�NaClHCl
��3���ƽ⣺����Ʒ�к�a2cO3 Ϊx�ˣ�ͬʱ����NaClΪy��

����:��1����ͼ���֪�����μ���73gϡ����ʱ������ȫ��Ӧ��������ų������������Ϊz�����ݻ�ѧ����ʽ�ɵ�737.3=44z����֮��z=4.4g���ʷų������������Ϊ4.4g��
��2����A��ʱ����ȫ��Ӧ���ʵ�B ��ʱ������ʣ�࣬���ձ�����Һ���������NaCl��HCl��
��3���ձ��ﲻ������Һ�����ʵ�����Ϊ��11.7g+��22.3g-10.6g��=23.4g
�ձ��ﲻ������Һ����Ϊ��22.3g+73g-4.4g=90.9g
�ձ��ﲻ������Һ�����ʵ���������Ϊ����23.4g��90.9g����100%=25.7%
���������ʵ���������Ϊ25.7%��

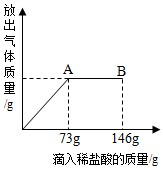 ��һ�ձ���ʢ��22.3gNa2CO3��NaCl��ɵĹ�������������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��һ�ձ���ʢ��22.3gNa2CO3��NaCl��ɵĹ�������������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺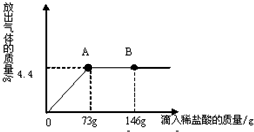
 ��һ�ձ���ʢ��22.3g Na2CO3��NaCl��ɵĹ��������143.1gˮ�ܽ⣬�Ƴ���Һ��
��һ�ձ���ʢ��22.3g Na2CO3��NaCl��ɵĹ��������143.1gˮ�ܽ⣬�Ƴ���Һ�� ��2013?��������һģ����һ�ձ���ʢ��22.3Na2CO3��NaCl��ɵĹ����������109.1g ˮʹ����ȫ�ܽ⣬�����Һ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��2013?��������һģ����һ�ձ���ʢ��22.3Na2CO3��NaCl��ɵĹ����������109.1g ˮʹ����ȫ�ܽ⣬�����Һ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺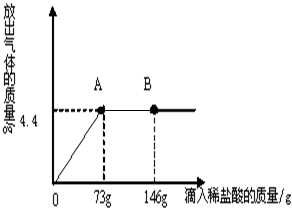 С���ڻ�ѧʵ���ҷ��֣�ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ��С�ս���С����С�죬��ͬ̽�����ְ�ɫ��ĩ�ijɷ֣�
С���ڻ�ѧʵ���ҷ��֣�ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ��С�ս���С����С�죬��ͬ̽�����ְ�ɫ��ĩ�ijɷ֣�