��Ŀ����
��ѧ��ȤС���ij��ҵ��ˮ������H2SO4��Na2SO4����H2SO4�ĺ������вⶨ���ס�����ͬѧ�������ͬ�IJⶨ������
��1����ͬѧ������кͷ�
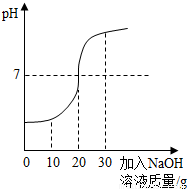
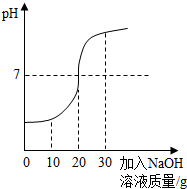
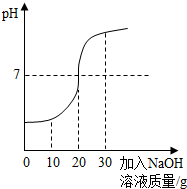
ȡ60g��ˮ���ձ��У�����������������Ϊ10%��NaOH��Һ����Ӧ�Ļ�ѧ����ʽΪ��H2SO4+2NaOH==Na2SO4+ 2H2O������Ӧ������ҺpH�仯����ͼ�� ��60g��ˮ��H2SO4��������
��2����ͬѧ������������
����BaCl2��Һ����NaOH��Һ�ⶨ��ˮ�е�H2SO4�ĺ�������Ӧ�Ļ�ѧ����ʽΪ��H2SO4+ BaCl2==BaSO4��+2HCl��������Ϊ����� ���ƫ�ߡ�����ƫ�͡��������䡱�������� ��
(1) 2.45g
��2��ƫ�ߣ�1�֣� BaCl2��ҺҲ�����ˮ�е�Na2SO4��Ӧ����2�֣�
����������1����PH=7ʱ����Һ�����ԣ�˵������ǡ����ȫ��Ӧ����ʱ�����NaOH��Һ������Ϊ20�ˡ�
��:���ˮ�����������Ϊx
H2SO4+2NaOH=Na2SO4+2H2O
98 80
x 20g��10%
98/80=X/20g��10% ��2�֣�
X=2.45g ��1�֣�
�𣺷�ˮ�����������Ϊ2.45g
��2��ƫ�ߣ���Ϊ BaCl2��ҺҲ�����ˮ�е�Na2SO4��Ӧ��

 ��2012?���£���ѧ��ȤС���ij��ҵ��ˮ������H2SO4��Na2SO4����H2SO4�ĺ������вⶨ���ס�����ͬѧ�������ͬ�IJⶨ������
��2012?���£���ѧ��ȤС���ij��ҵ��ˮ������H2SO4��Na2SO4����H2SO4�ĺ������вⶨ���ס�����ͬѧ�������ͬ�IJⶨ������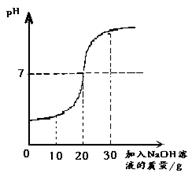
 ��ѧ��ȤС���ij��ҵ��ˮ������H2SO4��Na2SO4����H2SO4�ĺ������вⶨ���ס�����ͬѧ�������ͬ�IJⶨ������
��ѧ��ȤС���ij��ҵ��ˮ������H2SO4��Na2SO4����H2SO4�ĺ������вⶨ���ס�����ͬѧ�������ͬ�IJⶨ������