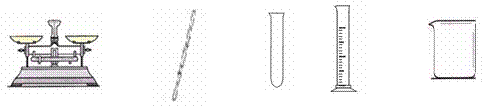
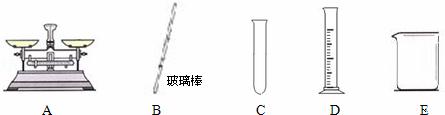
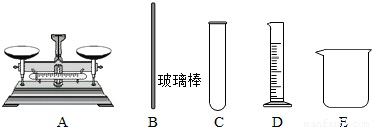
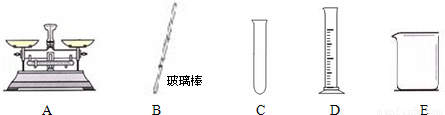
��Ŀ����
�����������һ��������������������������Һ���������йؼ��㡣
��1��С��ͬѧ��ʵ����������10%������������Һ143g����ش�
������ 10%������������Һ143g����Ҫ40%������������Һ���ܶ�Ϊ1.43g/cm3�� mL����ˮ mL
���������һλС������
��ʵ���У������õ��������� ��
��2����һ�������Ķ�����̼ͨ�� 200g10%������������Һ�У�ǡ����ȫ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ �����ɵ�̼���Ƶ������� g��
��1����25��107.3����BDE����2��2NaOH+CO2��Na2CO3+H2O��26.5
��������
�����������1����Һ���ƹ����У����ʵ��������ֲ��䣬���Ǽ���Ļ������������ݡ����ڲ��õ�������������Һ����������������ҺҲҪ��������������������ȡ�����ƹ����У����ڶ�����Һ�����Բ���������ƽ����������Ͳ���õ��ձ�����ϡ�ͣ�ϡ��������Ҫ���������н��衣
������10%����������143g������Ҫ��40%������������Һ������Ϊx����
������Һϡ��ǰ�����ʵ��������ֲ���ɵ�
10%��143g��40%��x
���x��35.75g
��Ӧ�������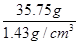 ��25cm3��25mL
��25cm3��25mL
�����ˮ������Ϊ143g��35.75g��107.25g��107.3g
��ˮ��Ӧ�����Ϊ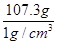 ��107.3cm3��107.3mL
��107.3cm3��107.3mL
������ϡ���̶���Һ�壬��������Ͳ������������ƽ��ϡ����Ҫ���ձ��н��У�ͬʱ��Ҫ���������裮����õ�������ΪBDE��
��2��������̼����������ǡ�÷�Ӧ�����ֿ��ܣ�����������ȫת��Ϊ̼���ƣ���������ת��Ϊ̼�����ƺ�̼���ƣ���������ת��Ϊ̼�����ƣ�����ĿҪ�����̼���Ƶ������������Ǽ���ֻ������̼���ƣ�������������ǡ�
��Ӧ�ķ�Ӧ����ʽΪ2NaOH+CO2��Na2CO3+H2O
��200g10%������������ȫת��Ϊ̼����ʱ�����ɵ�̼���Ƶ�����Ϊy
2NaOH+CO2��Na2CO3+H2O
80 106
200g��10% y
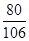 ��
��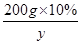
���y��26.5g
���㣺����һ������������������Һ������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�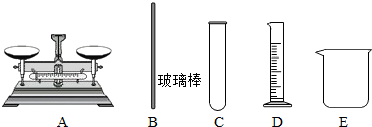
 ʵ���������� 10%������������Һ 143g����ش�
ʵ���������� 10%������������Һ 143g����ش�