��Ŀ����
����������������ˮ̨����ֱ��ȡˮ���á����е�����ˮ������������ͼ��ʾ��

��1�����л���̿�������� ����������ƹ���������� ��
��2������������ǧ������Դ����Ͷ�����У����в��ֹ��������ö�����(C2H6O)��ȼ�ϡ���������ȫȼ�����ɶ�����̼��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ ���������� ����3����Ȫˮ������ˮ�ж������ã����磺þ���Ļ��Ϸ�Ӧʮ�ֻ��������μ�����ˮ����Ӧ�������ҽ��У���Ӧ�ٶȴ����ߣ���ʱˮ���������������� ��
���𰸡�
��1������ ɱ������ ��2�֣�
��2��C2H6O
+ 3O2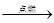 2CO2 + 3 H2O
��1�֣�
2CO2 + 3 H2O
��1�֣�
��3������ ��1�֣�
����������

��ϰ��ϵ�д�
�����Ŀ




 2010���Ϻ�������������ǡ����У�����������á���
2010���Ϻ�������������ǡ����У�����������á���
