��Ŀ����
һƿ�������ƹ��壬�������ڷ�����һ��ʱ�䣬�Ѿ����ֱ��ʡ���ѧ������ȤС���ͬѧ�����ⶨ��ƿ�Լ����ʵij̶ȣ�������֪ʶ�ع˵Ļ����ϣ����ν��������µ�ʵ�������
֪ʶ�عˣ��������Ʊ����ܷⱣ�棻���������������̼��Ӧ����Na2CO3��H2O��CaCO3������ˮ��
��һ����ȡ��ƿ�е��Լ�20g��ˮ���Ƴ���Һ��
�ڶ�������������Һ�м��������ij���ʯ��ˮ��
�����������ˡ�������������ɳ���Ϊ5g��
������ƿ�Լ��е�Na2CO3����������ΪA�����������Ƶ���������___________������ڡ����ڻ�С�ڣ�A��ԭ����__________________��
�Ƽ����20g�Լ��к�������Na2CO3�����������������ȷ��0.1%����
֪ʶ�عˣ��������Ʊ����ܷⱣ�棻���������������̼��Ӧ����Na2CO3��H2O��CaCO3������ˮ��
��һ����ȡ��ƿ�е��Լ�20g��ˮ���Ƴ���Һ��
�ڶ�������������Һ�м��������ij���ʯ��ˮ��
�����������ˡ�������������ɳ���Ϊ5g��
������ƿ�Լ��е�Na2CO3����������ΪA�����������Ƶ���������___________������ڡ����ڻ�С�ڣ�A��ԭ����__________________��
�Ƽ����20g�Լ��к�������Na2CO3�����������������ȷ��0.1%����
��С�ڣ��Լ��к���ˮ
�ƽ⣺���20g�Լ��к�̼���Ƶ�����Ϊx��
Na2CO3+Ca(OH)2==CaCO3��+2NaOH
��106��������������100
��x����������������5g
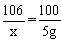
x=5.3g
��20g�Լ��к�̼���Ƶ���������Ϊ��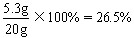
�ƽ⣺���20g�Լ��к�̼���Ƶ�����Ϊx��
Na2CO3+Ca(OH)2==CaCO3��+2NaOH
��106��������������100
��x����������������5g
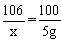
x=5.3g
��20g�Լ��к�̼���Ƶ���������Ϊ��

��ϰ��ϵ�д�
�����Ŀ
 һƿ�������ƹ��壬�������ڷ�����һ��ʱ�䣬�Ѿ����ֱ��ʣ���ѧ������ȤС���ͬѧ�����ⶨ��ƿ�Լ����ʵij̶ȣ�������֪ʶ�ع˵Ļ����ϣ����ν��������µ�ʵ�������
һƿ�������ƹ��壬�������ڷ�����һ��ʱ�䣬�Ѿ����ֱ��ʣ���ѧ������ȤС���ͬѧ�����ⶨ��ƿ�Լ����ʵij̶ȣ�������֪ʶ�ع˵Ļ����ϣ����ν��������µ�ʵ�������