��Ŀ����
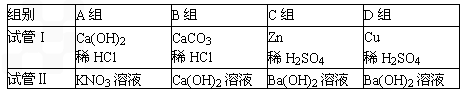
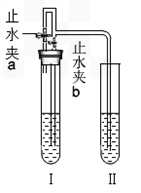
ij��ѧС��ͬѧ����ͼ��ʾװ�úͱ��������Լ�����ʵ�飨ͼ������̨�ȼг�����������ȥ����
��1�����Թ�I�м����Լ���������Ƥ�����ر�ֹˮ��a����ֹˮ��b���Թܢ���Һ����������Һ����ǣ���������ʵ��������Լ���______�飬��ʱ���з�����Ӧ�Ļ�ѧ����ʽΪ______���������������ԭ����______��
��2�����Թ�I�м����Լ���������Ƥ����������ֹˮ��a���ر�ֹˮ��b���Թܢ���������ð������Һ����ǣ���������ʵ��������Լ���______�飮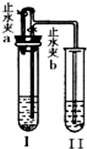
| ��� | A�� | B�� | C�� | D�� |
| �Թ�I | Ca��OH��2 ϡHCl |
CaCO3 ϡHCl |
Zn ϡH2SO4 |
Cu ϡH2SO4 |
| �Թܢ� | KNO3��Һ | Ca��OH��2��Һ | Ba��OH��2��Һ | Ba��OH��2��Һ |
��2�����Թ�I�м����Լ���������Ƥ����������ֹˮ��a���ر�ֹˮ��b���Թܢ���������ð������Һ����ǣ���������ʵ��������Լ���______�飮

A���������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ�����е�ѹǿ���䣬�������Һ�������У�B��̼��ƺ����ᷴӦ���ɶ�����̼���壬������̼���������У�ʹ����������Һ����ǣ�C��п�����ᷴӦ��������п��������ʹ����ѹǿ�������ɵ�����п��ѹ����У�������������Ӧ�������ᱵ������ʹ��Һ����ǣ�D��ͭ��ϡ�����Ӧ�����
��1��C��Ba��OH��2+H2SO4=BaSO4��+2H2O��Zn��ϡH2SO4��Ӧ����H2ʹI����Һ�ŵ�����ʹҺ������������Һ��Ϸ�����Ӧ���ɲ�����ˮ��BaSO4ʹ��Һ����ǣ�
��2��B��
��1��C��Ba��OH��2+H2SO4=BaSO4��+2H2O��Zn��ϡH2SO4��Ӧ����H2ʹI����Һ�ŵ�����ʹҺ������������Һ��Ϸ�����Ӧ���ɲ�����ˮ��BaSO4ʹ��Һ����ǣ�
��2��B��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
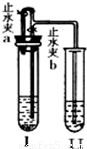
ij��ѧС��ͬѧ����ͼ��ʾװ�úͱ��������Լ�����ʵ�飨ͼ������̨�ȼг�����������ȥ����
��1�����Թ�I�м����Լ���������Ƥ�����ر�ֹˮ��a����ֹˮ��b���Թܢ���Һ����������Һ����ǣ���������ʵ��������Լ���______�飬��ʱ���з�����Ӧ�Ļ�ѧ����ʽΪ______���������������ԭ����______��
��2�����Թ�I�м����Լ���������Ƥ����������ֹˮ��a���ر�ֹˮ��b���Թܢ���������ð������Һ����ǣ���������ʵ��������Լ���______�飮
| ��� | A�� | B�� | C�� | D�� |
| �Թ�I | Ca��OH��2 ϡHCl | CaCO3 ϡHCl | Zn ϡH2SO4 | Cu ϡH2SO4 |
| �Թܢ� | KNO3��Һ | Ca��OH��2��Һ | Ba��OH��2��Һ | Ba��OH��2��Һ |
��2�����Թ�I�м����Լ���������Ƥ����������ֹˮ��a���ر�ֹˮ��b���Թܢ���������ð������Һ����ǣ���������ʵ��������Լ���______�飮


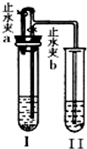 ij��ѧС��ͬѧ����ͼ��ʾװ�úͱ��������Լ�����ʵ�飨ͼ������̨�ȼг�����������ȥ����
ij��ѧС��ͬѧ����ͼ��ʾװ�úͱ��������Լ�����ʵ�飨ͼ������̨�ȼг�����������ȥ����