��Ŀ����
����淋Ļ�ѧʽΪ��NH4��2SO4���Լ��㣺[�ڣ�3������4��д���������]
��1������淋���Է�������
��2����������У������⡢��������Ԫ�ص�������Ϊ
��3��100kg��������е�Ԫ�ص�����Ϊ���٣�
��4��ijũ��ƻ�ʩ��200kg������[CO��NH2��2]���������ڻ�Դ��ȱ�����������[��NH4��2SO4]��Ϊ��֤�������൱����������淋������Ƕ��٣�
��1������淋���Է�������
132
132
��2����������У������⡢��������Ԫ�ص�������Ϊ
7��2��8��16
7��2��8��16
��3��100kg��������е�Ԫ�ص�����Ϊ���٣�
��4��ijũ��ƻ�ʩ��200kg������[CO��NH2��2]���������ڻ�Դ��ȱ�����������[��NH4��2SO4]��Ϊ��֤�������൱����������淋������Ƕ��٣�
��������1��������Է�������Ϊ��ѧʽ�и�ԭ�ӵ����ԭ������֮�ͣ����з������
��2�����ݻ������и�Ԫ��������=��ԭ�ӵ����ԭ��������ԭ�Ӹ���֮�ȣ����з������
��3����������淋�������������е�Ԫ�ص������������㣮
��4�����ݻ�������ijԪ�ص�����=�û��������������Ԫ�ص��������������з������
��2�����ݻ������и�Ԫ��������=��ԭ�ӵ����ԭ��������ԭ�Ӹ���֮�ȣ����з������
��3����������淋�������������е�Ԫ�ص������������㣮
��4�����ݻ�������ijԪ�ص�����=�û��������������Ԫ�ص��������������з������
����⣺��1������淋���Է�����������14+1��4����2+32+16��4=132��
��2��������е����⡢��������Ԫ�ص�������Ϊ��14��2������1��8����32����16��4��=7��2��8��16��
��3��100kg��������е�Ԫ�ص�����Ϊ��100kg��
��100%=21.2kg��
��4������Ҫ����淋�����Ϊx����
200kg��
��100%=x��
��100%100% ��ã�x=420kg��
�ʴ�Ϊ����1������淋���Է������� 132����2����������У������⡢��������Ԫ�ص�������Ϊ 7��2��8��16����3��21.2kg����4��420kg��
��2��������е����⡢��������Ԫ�ص�������Ϊ��14��2������1��8����32����16��4��=7��2��8��16��
��3��100kg��������е�Ԫ�ص�����Ϊ��100kg��
| 14��2 |
| 132 |
��4������Ҫ����淋�����Ϊx����
200kg��
| 14��2 |
| 14��2+1��4+16��3 |
| 14��2 |
| 132 |
�ʴ�Ϊ����1������淋���Է������� 132����2����������У������⡢��������Ԫ�ص�������Ϊ 7��2��8��16����3��21.2kg����4��420kg��
�����������ѶȲ�����ͬѧ�ǽ������Ϣ��������û�ѧʽ���йؼ�����з������⡢��������������

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�����Ŀ
ij������Ϊ�ۺ��������������еĸ���ƷCaSO4�������ڵĻ��ʳ�����������Ʊ���NH4��2SO4�Ĺ������̣�
���������̼�ԭ����
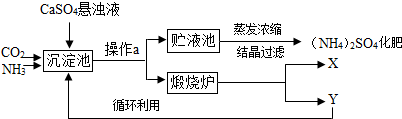
�������з�������Ҫ��ѧ��ӦΪ��CO2+2NH3+CaSO4+H2O=CaCO3��+��NH4��2SO4
�����۽�����
��1������a�������� ������ʵ���ҽ��д˲������õ��IJ������������������ձ��⣬����Ҫ ��
��2��������a��õ��Ĺ�������������¯�и��·ֽ�����X��Y�������ʣ��ù����У�Y����Ϊԭ��֮һѭ�����ã�Y�Ļ�ѧʽΪ ��
��3����������Ҳ������������NH4HCO3���÷�Ӧ�Ļ�ѧ����ʽΪ ��
������炙��ʵȼ��ⶨ��
��1���жϣ�NH4��2SO4����������NH4HCO3��
ȡ������������ˮ���μ������� �������ݲ���������жϸû����в�����NH4HCO3��
��2�������е�Ԫ�غ����IJⶨ����ȷ���û����в�����NH4HCO3���ʣ���
���������ϡ�
����֪����NH4��2SO4+2NaOH
Na2SO4+2NH3��+2H2O
������������ˮ����ˮ��ҺΪ��ˮ����ˮ�ʼ��ԣ����ȶ����ӷ���
�ڹ�ҵ�ж�����炙��ʵȼ�ָ�����£�
��ʵ����ơ�
����ͼ��ʾװ�ý���ʵ�飮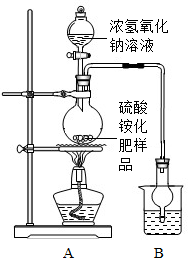
��1��ʵ������У�����ƿ�м�������Ũ����������Һ��������ʹ����麟�ַ�Ӧ��ȫת��ΪNH3��
��2���ձ��е��Լ���Ϊ�����ղ����İ��������������˵��Լ��� ������ţ���
��ˮ����ŨHCl����ϡH2SO4����NaOH��Һ
�ձ��и���ܵ������� ��
���������ۡ�
ʵ��С���ͬѧ��Ϊ��������ʵ��װ�ò�õĽ�����ܻ�������������
��1����ͬѧ��Ϊ��Ӧ��ʵ��װ��A��B֮������һ��װ�� ���Ũ���ᡰ��ʯ�ҡ����ĸ���װ�ã�����ʵ���õ�����炙��ʺ���������ƫ�ߣ�
��2����ͬѧ��Ϊ����ʵ��װ��A��B֮��������ȷ�ĸ���װ�ú����ʵ�飬��õ�����炙��ʺ���������ƫ�ͣ������� ��
��ʵ��ⶨ��
�����ۺ����ǸĽ���ʵ��װ�ã��ų��˿��ܲ����������أ����½���ʵ�飮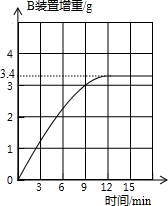
ȡ����炙�����Ʒ13.5 g����ʵ�飬���Bװ�������뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ����ͨ�������жϸû��ʵĵȼ���
������̣�
�û��ʵĵȼ�Ϊ Ʒ��
���������̼�ԭ����

�������з�������Ҫ��ѧ��ӦΪ��CO2+2NH3+CaSO4+H2O=CaCO3��+��NH4��2SO4
�����۽�����
��1������a��������
��2��������a��õ��Ĺ�������������¯�и��·ֽ�����X��Y�������ʣ��ù����У�Y����Ϊԭ��֮һѭ�����ã�Y�Ļ�ѧʽΪ
��3����������Ҳ������������NH4HCO3���÷�Ӧ�Ļ�ѧ����ʽΪ
������炙��ʵȼ��ⶨ��
��1���жϣ�NH4��2SO4����������NH4HCO3��
ȡ������������ˮ���μ�������
��2�������е�Ԫ�غ����IJⶨ����ȷ���û����в�����NH4HCO3���ʣ���
���������ϡ�
����֪����NH4��2SO4+2NaOH
| ||
������������ˮ����ˮ��ҺΪ��ˮ����ˮ�ʼ��ԣ����ȶ����ӷ���
�ڹ�ҵ�ж�����炙��ʵȼ�ָ�����£�
| ָ�� ��Ŀ |
�ŵ�Ʒ | �ϸ�Ʒ |
| ����N������ | ��21.0% | ��20.5% |
����ͼ��ʾװ�ý���ʵ�飮

��1��ʵ������У�����ƿ�м�������Ũ����������Һ��������ʹ����麟�ַ�Ӧ��ȫת��ΪNH3��
��2���ձ��е��Լ���Ϊ�����ղ����İ��������������˵��Լ���
��ˮ����ŨHCl����ϡH2SO4����NaOH��Һ
�ձ��и���ܵ�������
���������ۡ�
ʵ��С���ͬѧ��Ϊ��������ʵ��װ�ò�õĽ�����ܻ�������������
��1����ͬѧ��Ϊ��Ӧ��ʵ��װ��A��B֮������һ��װ��
��2����ͬѧ��Ϊ����ʵ��װ��A��B֮��������ȷ�ĸ���װ�ú����ʵ�飬��õ�����炙��ʺ���������ƫ�ͣ�������
��ʵ��ⶨ��
�����ۺ����ǸĽ���ʵ��װ�ã��ų��˿��ܲ����������أ����½���ʵ�飮

ȡ����炙�����Ʒ13.5 g����ʵ�飬���Bװ�������뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ����ͨ�������жϸû��ʵĵȼ���
������̣�
�û��ʵĵȼ�Ϊ
