��Ŀ����
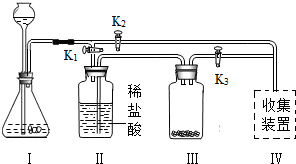
 32����ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�飮Aװ����װ�������ԼΪ3��1�Ŀ����Ͷ�����̼�Ļ������Bװ����ʢ���������ۣ�Cװ����ʢ��������ϡ���ᣮ
32����ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�飮Aװ����װ�������ԼΪ3��1�Ŀ����Ͷ�����̼�Ļ������Bװ����ʢ���������ۣ�Cװ����ʢ��������ϡ���ᣮ��1���رջ���K������Aװ���н�ͷ�ι�������������������Һ����ƿ�У����Թ۲쵽Aװ���е�������
�������
��Aװ�÷�����Ӧ�Ļ�ѧ����ʽ2NaOH+CO2=Na2CO3+H2O
����2����������K��һ��ʱ��رջ���K��������ʵ������У����Թ۲쵽Cװ���е�������
��Һ�ӵ���������Һ���½�������©���¶������ݷų�
�����ܿ������ݷų���Һ���½�������©����Һ������
����3��Cװ�õ�������
acd
������ĸ����a���ṩҩƷ b�������������� c������װ���ڵ�ѹǿ d���������������壮
��������1����������������Һ�������̼��Ӧʹװ����ѹǿ��С���ٸ���ʵ��װ���������������ʲô����Ҫ��ϵ��������ѹ����װ���ڵ�ѹ���
��2����Aװ������ѹ��С������Bװ������ѹҲ��С����������ѹ���������ٷ�����Ŀ��
��3��Cװ��ͨ������װ���ڵ�ѹǿ���ṩҩƷ��ͬʱҲ�����������������
��2����Aװ������ѹ��С������Bװ������ѹҲ��С����������ѹ���������ٷ�����Ŀ��
��3��Cװ��ͨ������װ���ڵ�ѹǿ���ṩҩƷ��ͬʱҲ�����������������
����⣺��1�������������������̼��Ӧ���װ������ѹ��С����������ѹ�������£�������������������ͣ�
��2����Aװ������ѹ��С������Bװ������ѹҲ��С����������ѹ�������£�Cװ���е�ϡ��������Bװ���У��������ᷴӦ�����٣����������ݣ���Һ����ɫ���dz��ɫ��
��3�����ڴ���ѹ�������±�ѹ��Bװ�ã����з�Ӧ������Cװ��ͨ������װ���ڵ�ѹǿ���ṩҩƷ��ͬʱҲ�����������������
�ʴ�Ϊ����1�����������ʹ�2NaOH+CO2=Na2CO3+H2O
��2����Һ�ӵ���������Һ���½�������©���¶������ݷų������ܿ������ݷų���Һ���½�������©����Һ��������
��3��acd
��2����Aװ������ѹ��С������Bװ������ѹҲ��С����������ѹ�������£�Cװ���е�ϡ��������Bװ���У��������ᷴӦ�����٣����������ݣ���Һ����ɫ���dz��ɫ��
��3�����ڴ���ѹ�������±�ѹ��Bװ�ã����з�Ӧ������Cװ��ͨ������װ���ڵ�ѹǿ���ṩҩƷ��ͬʱҲ�����������������
�ʴ�Ϊ����1�����������ʹ�2NaOH+CO2=Na2CO3+H2O
��2����Һ�ӵ���������Һ���½�������©���¶������ݷų������ܿ������ݷų���Һ���½�������©����Һ��������
��3��acd
������ͨ���ش����֪��Щ��Ŀ��Ҫ�������֪ʶ���ش𣬲�������������ѹ��ƿ�ڵ�ѹ���������⣮

��ϰ��ϵ�д�
�����Ŀ
 ��ʦ����ͼ��ʾװ��Ϊͬѧ������������Ȥʵ�飮
��ʦ����ͼ��ʾװ��Ϊͬѧ������������Ȥʵ�飮

 ��2012?������һģ����ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�飮Aװ�ü���ƿ��װ�������ԼΪ1��1�ĵ���������a�Ļ�����壬ע������װ����������ɫ��Һb��Bװ����ʢ��������ɫʯ����Һ��Cװ����ʢ�����������������Dװ����ʢ��������ϡ���ᣮ
��2012?������һģ����ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�飮Aװ�ü���ƿ��װ�������ԼΪ1��1�ĵ���������a�Ļ�����壬ע������װ����������ɫ��Һb��Bװ����ʢ��������ɫʯ����Һ��Cװ����ʢ�����������������Dװ����ʢ��������ϡ���ᣮ