��Ŀ����
(1)��ҵ��Ϊ�˲ⶨ�����Ũ��(���ʵ���������)������һ����Ũ�ȵ�����������Һ�����ᷴӦ����ѧ��Ӧ����ʽ�� ��
(2)���ȣ�������������Ϊ10%������������Һ120g����Ҫ g�������ƹ����ˮ g��
(3)������������Ϊ10%������������Һ�����������ͼ����ȷ�IJ���˳���� ��
(2)���ȣ�������������Ϊ10%������������Һ120g����Ҫ g�������ƹ����ˮ g��
(3)������������Ϊ10%������������Һ�����������ͼ����ȷ�IJ���˳���� ��

(4)��������õ�10%������������Һ40g�����ᷴӦ���Է�̪��ָʾ���������������Һ����ɫ�ɺ�ɫ�պñ�Ϊ��ɫʱ����¼��Ӧ�к���������Һ����Ϊ98g������������������������ ��
(1)2NaOH+H2SO4==Na2SO4+2H2O
(2) 12��108
(3) �ܢݢ٢ڢ�
(4) 5%
(2) 12��108
(3) �ܢݢ٢ڢ�
(4) 5%

��ϰ��ϵ�д�
�����Ŀ
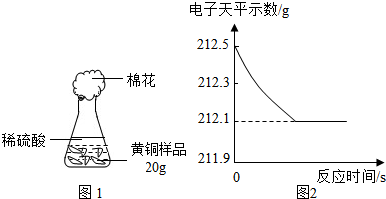
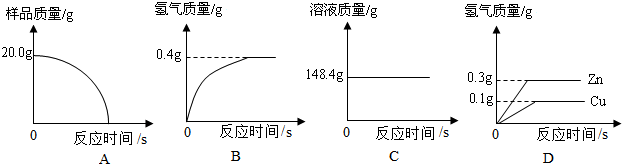
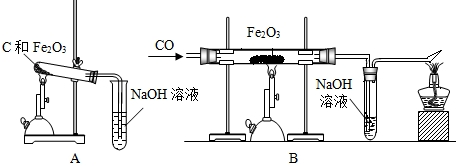
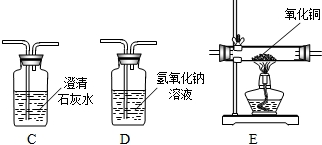
 14����ˮ����������������������أ���ش��������⣺
14����ˮ����������������������أ���ش��������⣺