��Ŀ����
Ϊ�˲ⶨ����ʯ��
CaCO3������������ȡ����ʯ12.5g������ʢ��100gϡ������ձ��У�����ǡ����ȫ��Ӧ(�����������ʲ������ᷴӦ��Ҳ���ܽ�)���ձ�������������Ϊ108.1g��(1)��Ӧ�Ļ�ѧ����ʽ��________��
(2)����CO2����________g��
(3)12.5g����ʯ��CaCO3________g������ʯ��CaCO3����������Ϊ________g��
(4)100gϡ�����е�������________����������________g��
(5)��ȫ��Ӧ���ձ��ڻ���________g�������ʣ��ձ������ɵ���Һ��________��Һ��������Һ������Ϊ________g���������ʵ�����Ϊ________g���ܼ�������Ϊ________g��
�𰸣�
������
������
|
���� (1)CaCO3��2HCl��CaCl2��H2O��CO2������ (2)4.4���� (3)10,80������ (4)HCl(�Ȼ�������),7.3���� (5)2.5,�Ȼ���,105.6,11.1,94.5 |

��ϰ��ϵ�д�
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
�����Ŀ
CaCO3�㷺��������Ȼ�磬��һ����Ҫ�Ļ���ԭ�ϣ�ij�ִ���ʯ����Ҫ�ɷ�ΪCaCO3�⣬�������������С���С��ͬѧ�����ִ���ʯ��ϡ���ᷴӦ���ֱ�չ����̽�����������̽�����ش�������⣮
���������ϡ�
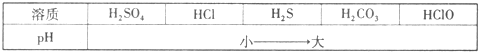
����һ����֪���ֽⷴӦCaCO3+2HCI=CO2��+H2O+CaCl2���Է����У��ڳ����£����Ũ�Ⱦ�Ϊa%������������Һ��pH��С�����
����pH��С�����ʾ�����ֽⷴӦ��һ�����ɣ�����ǿ�ᷢ�����Ʒ�Ӧ�������ɽ����ᣮ���з�Ӧ���ܷ��������в����ϸù��ɵ���______������ĸ����
A��H2SO4+2NaHCO3=2NaCl+2H2O+2CO2�� B��2HCl+CaS=CaCl2+H2S��
C��CO2+H2O+Ca��ClO��2=CaCO3��+2HClO D��H2S+CuSO4=H2SO4+CuS��
���϶�������������Т�Ũ�������ˮ�Ȼ��Ƣۼ�ʯ�Ң���ʯ�Ң�����������
��ʵ��̽����
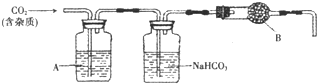
С��ͬѧΪ�˵õ������Ķ�����̼�����������װ�ã����������

��1���Ʊ���CO2�����У����ܺ��е�������______��
��2������װ���У�A��______��Һ��NaHCO3��Һ��������______��
��3������װ���У�B���ʵ����ƿ�����______��
��4�������ʵ��õ�������ⶨCO2����Է������������B����ʧЧ���ⶨ���______ ���ƫ�ߡ�����ƫ�͡�����Ӱ�조����
��5��С��ͬѧΪ�˼������ִ���ʯ�к�̼����������������������¶���ʵ�飺
С��ͬѧͨ���������㣬�ó����ۣ���ʵ�������ɵĶ�����̼��������4.4g������ʯ��Ʒ����Ϊ83.3%�������������Ľ����Ƿ���ȷ______�����ȷ������������������______��
���������ϡ�
����һ����֪���ֽⷴӦCaCO3+2HCI=CO2��+H2O+CaCl2���Է����У��ڳ����£����Ũ�Ⱦ�Ϊa%������������Һ��pH��С�����
| ���� | H2SO4 | HCl | H2S | H2CO3 | HClO |
| pH | ���� | ||||
A��H2SO4+2NaHCO3=2NaCl+2H2O+2CO2�� B��2HCl+CaS=CaCl2+H2S��
C��CO2+H2O+Ca��ClO��2=CaCO3��+2HClO D��H2S+CuSO4=H2SO4+CuS��
���϶�������������Т�Ũ�������ˮ�Ȼ��Ƣۼ�ʯ�Ң���ʯ�Ң�����������
��ʵ��̽����
С��ͬѧΪ�˵õ������Ķ�����̼�����������װ�ã����������

��1���Ʊ���CO2�����У����ܺ��е�������______��
��2������װ���У�A��______��Һ��NaHCO3��Һ��������______��
��3������װ���У�B���ʵ����ƿ�����______��
��4�������ʵ��õ�������ⶨCO2����Է������������B����ʧЧ���ⶨ���______ ���ƫ�ߡ�����ƫ�͡�����Ӱ�조����
��5��С��ͬѧΪ�˼������ִ���ʯ�к�̼����������������������¶���ʵ�飺
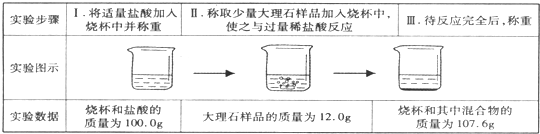
| ʵ�鲽�� | I����������������ձ��в����� | II����ȡ��������ʯ��Ʒ�����ձ��У�ʹ֮�����ϡ���ᷴӦ | III������ȫ��Ӧ���� |
| ʵ��ͼʾ |  | ||
| ʵ������ | �ձ������������Ϊ100.0g | ����ʯ��Ʒ������Ϊ12.0g | �ձ������л���������Ϊ107.6g |
CaCO3�㷺��������Ȼ�磬��һ����Ҫ�Ļ���ԭ�ϣ�ij�ִ���ʯ����Ҫ�ɷ�ΪCaCO3�⣬�������������С���С��ͬѧ�����ִ���ʯ��ϡ���ᷴӦ���ֱ�չ����̽�����������̽�����ش�������⣮
���������ϡ�
����һ����֪���ֽⷴӦCaCO3+2HCI=CO2��+H2O+CaCl2���Է����У��ڳ����£����Ũ�Ⱦ�Ϊa%������������Һ��pH��С�����
����pH��С�����ʾ�����ֽⷴӦ��һ�����ɣ�����ǿ�ᷢ�����Ʒ�Ӧ�������ɽ����ᣮ���з�Ӧ���ܷ��������в����ϸù��ɵ���______������ĸ����
A��H2SO4+2NaHCO3=2NaCl+2H2O+2CO2�� B��2HCl+CaS=CaCl2+H2S��
C��CO2+H2O+Ca��ClO��2=CaCO3��+2HClO D��H2S+CuSO4=H2SO4+CuS��
���϶�������������Т�Ũ�������ˮ�Ȼ��Ƣۼ�ʯ�Ң���ʯ�Ң�����������
��ʵ��̽����
С��ͬѧΪ�˵õ������Ķ�����̼�����������װ�ã����������

��1���Ʊ���CO2�����У����ܺ��е�������______��
��2������װ���У�A��______��Һ��NaHCO3��Һ��������______��
��3������װ���У�B���ʵ����ƿ�����______��
��4�������ʵ��õ�������ⶨCO2����Է������������B����ʧЧ���ⶨ���______ ���ƫ�ߡ�����ƫ�͡�����Ӱ�조����
��5��С��ͬѧΪ�˼������ִ���ʯ�к�̼����������������������¶���ʵ�飺
С��ͬѧͨ���������㣬�ó����ۣ���ʵ�������ɵĶ�����̼��������4.4g������ʯ��Ʒ����Ϊ83.3%�������������Ľ����Ƿ���ȷ______�����ȷ������������������______��
���������ϡ�
����һ����֪���ֽⷴӦCaCO3+2HCI=CO2��+H2O+CaCl2���Է����У��ڳ����£����Ũ�Ⱦ�Ϊa%������������Һ��pH��С�����
| ���� | H2SO4 | HCl | H2S | H2CO3 | HClO |
| pH | ���� | ||||
A��H2SO4+2NaHCO3=2NaCl+2H2O+2CO2�� B��2HCl+CaS=CaCl2+H2S��
C��CO2+H2O+Ca��ClO��2=CaCO3��+2HClO D��H2S+CuSO4=H2SO4+CuS��
���϶�������������Т�Ũ�������ˮ�Ȼ��Ƣۼ�ʯ�Ң���ʯ�Ң�����������
��ʵ��̽����
С��ͬѧΪ�˵õ������Ķ�����̼�����������װ�ã����������

��1���Ʊ���CO2�����У����ܺ��е�������______��
��2������װ���У�A��______��Һ��NaHCO3��Һ��������______��
��3������װ���У�B���ʵ����ƿ�����______��
��4�������ʵ��õ�������ⶨCO2����Է������������B����ʧЧ���ⶨ���______ ���ƫ�ߡ�����ƫ�͡�����Ӱ�조����
��5��С��ͬѧΪ�˼������ִ���ʯ�к�̼����������������������¶���ʵ�飺
| ʵ�鲽�� | I����������������ձ��в����� | II����ȡ��������ʯ��Ʒ�����ձ��У�ʹ֮�����ϡ���ᷴӦ | III������ȫ��Ӧ���� |
| ʵ��ͼʾ |  | ||
| ʵ������ | �ձ������������Ϊ100.0g | ����ʯ��Ʒ������Ϊ12.0g | �ձ������л���������Ϊ107.6g |