��Ŀ����
��3�֣��о���ѧϰС��Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ��õ�����ƽ�ֱ�Ƶ���ƿ����������Ϊ44.1g����ȡ��ͭ��Ʒ20.0g������ƿ�м������Ʒ������ϡ�����ƿ������������ͼ1��ʾ����������ƽ���������ݻ����ͼ2��


ͼ1 ͼ2
������������ݣ��ش��������⣺
��1���ĸ�ͬѧ�Ӷ�Ƕȴ������ݣ��������ݴ�����ͼ������ȷ���� ��

��2���Լ��㣺
����Ʒ��ͭ������������
��ǡ�÷�Ӧʱ������Һ�������������������
��1��B ��2��35%�� 20%
��������
�����������1�����ݾ���ķ�Ӧ�����Ƚϡ�
A����Ϊͭ����ϡ���ᷴӦ����ͭ��ֻ��п��ϡ���ᷴӦ��������ȫ��Ӧ����Ʒ��ʣ�ࣨͭ������A����
B�����������غ㶨�ɿɵã���ȫ��Ӧʱ��������������Ϊ��212.5g-212.1g=0.4g����B��ȷ��
C����Ϊп��ϡ���ᷴӦ���������������ɣ�������������С�ڲ��뷴Ӧ��п������������Һ���������ŷ�Ӧ�Ľ��ж������ӣ�����Ӧ��ȫʱ���ٱ仯����C����
D����Ϊͭ����ϡ���ᷴӦ����ͭ��ֻ��п��ϡ���ᷴӦ����D����ѡB��
��2������п�����ᷴӦ�Ļ�ѧ����ʽ�������������������г�����ʽ���Ϳɼ������Ʒ��п�����������ɵ�����п������������Ʒ��ͭ������=��Ʒ����-��Ʒ��п��������Ȼ����ݡ� ��100%�����㼴�ɣ���������Һ����������������������ɵ�����п����������Һ����=��ȫ��Ӧ�������ƽʾ��-��ƿ����������-ͭ��������Ȼ�������������������ʽ���㼴�ɡ�
��100%�����㼴�ɣ���������Һ����������������������ɵ�����п����������Һ����=��ȫ��Ӧ�������ƽʾ��-��ƿ����������-ͭ��������Ȼ�������������������ʽ���㼴�ɡ�
������Ʒ��п������Ϊx�����ɵ�����п������Ϊy
Zn + H2SO4 �� ZnSO4 + H2��
65 161 2
X y 0.4g


��֮�ã�X��13g y��32.2g
��Cu����������Ϊ 
��������Һ���������������������Ϊ ��100%��20%
��100%��20%
����Ʒ��ͭ����������Ϊ35%����Ӧ��������Һ������п����������Ϊ20%��
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ��㣬�й��������������ļ��㣬�����Ļ�ѧ����
������������Ҫ����ѧ�����ۺϷ����������ж����ݴ�����ͼ�������Ҫ�������ʼ䷴Ӧ�������ͼʾ���ݷ���������ʱҪ������֪�����ͻ�ѧ����ʽ�еĹ̶������ȣ��Լ�����������ʽ���з������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�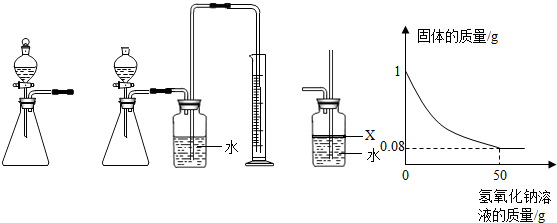
 ��2013?��ͨһģ������۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����
��2013?��ͨһģ������۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����
 ����۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����
����۳�Ϊ������ϲ����Ȼ��һЩ���������ñ��Ƿ�ð����������ۣ�һ�о���ѧϰС��Ϊ�˼���ij����۵���٣�����������̽����