��Ŀ����
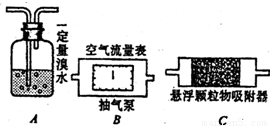
����������ɫ�д̼�����ζ���壩�������������������������Ҫ�Ĵ�����Ⱦ��ҹ�����ʮ�����ӻ��������������������̨ÿ�조������������Ҫ����ȫ��42����Ҫ���п��������ձ������������ǹ�ע�Լ����������Ŀǰ���йز��Ÿ���SO2��Br2���壩��H2O�Ķ�����Ӧ���ⶨ������SO2�ĺ�����������һԭ����ͨ���ڵ����з�����Ӧʱ�����ı仯������ȷ�ⶨ����������������ĺ�����ijУ��ѧ����С��Ϊ�ⶨУ����������SO2������������ĺ���������������Ӧԭ�����������װ�ã���1��������������ȷ�ⶨ�������õĿ����������ⶨ���������P����������ʱ����ͼ��ʾ����װ�����ӵ�˳���ǣ�����ţ�______��
��2��Ϊȷ�ⶨSO2�ĺ�����ʵ��ʱ���۲쵽______ʱ��Ӧ�����رճ����ã���Ӧԭ����
Br2+2H2O+SO2=H2SO4+2HBr
����ɫ�� ����ɫ��
��3����Ҫ�ⶨ����������������ĺ�������Ҫ���ʵ��ʱ���������⣬��Ҫ��______������ţ���
a��ʵ��ǰB������ b��ʵ���B ������
c��ʵ��ǰC ������ d..ʵ���C ��������
���𰸡���������������ṩ����Ϣ���з�����Ҫ�ֱ�ⶨ������������SO2����ʱ�������ÿ��ڱ��������ǰ�棬����������ʹ��ˮ��ɫ������������ĺ��������������������������仯�йأ�
����⣺��1��Ҫ�ⶨ������������SO2��������ʹ������������SO2���������ã������ÿ��ڱ��������ǰ�棬���Ա����Ϊ��BCA��
��2������������ʹ��ˮ��ɫ������ɫǡ����ȥʱ����������ǡ�÷�Ӧ�����Ա����Ϊ��A�е���ˮ�ɻ�ɫ��Ϊ��ɫ��
��3������������ĺ��������������������������仯�йأ����Ա����Ϊ��cd��
�ʴ�Ϊ����1��B��C��A�� ��2��A�е���ˮ�ɻ�ɫ��Ϊ��ɫ�� ��3��cd��
���������⿼���˿�����Ⱦ���й�֪ʶ����ɴ��⣬������������ṩ����Ϣ��������е�֪ʶ�����
����⣺��1��Ҫ�ⶨ������������SO2��������ʹ������������SO2���������ã������ÿ��ڱ��������ǰ�棬���Ա����Ϊ��BCA��
��2������������ʹ��ˮ��ɫ������ɫǡ����ȥʱ����������ǡ�÷�Ӧ�����Ա����Ϊ��A�е���ˮ�ɻ�ɫ��Ϊ��ɫ��
��3������������ĺ��������������������������仯�йأ����Ա����Ϊ��cd��
�ʴ�Ϊ����1��B��C��A�� ��2��A�е���ˮ�ɻ�ɫ��Ϊ��ɫ�� ��3��cd��
���������⿼���˿�����Ⱦ���й�֪ʶ����ɴ��⣬������������ṩ����Ϣ��������е�֪ʶ�����

��ϰ��ϵ�д�
�����Ŀ