��Ŀ����
����Ŀ�����������ķ�չ����ͥ�ջ���Ʒ������Ҳ�������࣬��ͼ��ʾ�Ǽ��ֳ����ļ���������
���� | 84����Һ | ����� | �ܵ�ͨ | ��ƯҺ |
��Ʒʾ�� |
|
|
|
|
��Ч�ɷ� | ��������(NaClO) | ����(HCl) | ��������( NaOH ) | ��������(H2O2) |
(1)���������������������_____(д��Ч�ɷֵ��������ƻ�ѧʽ)��
(2)�������������Ч�ɷ���ϡ���ᣬ������84����Һ�����ã��ײ���һ���ж����塣�÷�Ӧ�Ļ�ѧ����ʽΪNaClO+2HCl�TNaCl++H2O +X ������X�Ļ�ѧʽΪ____����������������ڴ���ʯ�����ϻ�����������ݣ�д���˷�Ӧ�Ļ�ѧ����ʽ____��
(3)���ܵ�ͨ����ʹ�����Ĺܵ���ͨ���裬��ܵ���ͨ������Ч�ɷ�Ϊ�������ƣ���ʹ��ʱ��ֹ��Ƥ���Ӵ�����ԭ����____����ʹ��ʱҲ������������������ʹ�ã���ԭ����____(�û�ѧ����ʽ��ʾ)��
(4)����Ư������ʹ��ʱ���ͷų�����ʹ���ձ��������ϴ�ӣ��䷴Ӧԭ����____(д��ѧ��Ӧ����ʽ)��
���𰸡�H2O2 Cl2 CaCO3+2HCl=CaCl2+CO2��+H2O ����������ǿ�ҵĸ�ʴ�� NaOH+HCl==NaCl+H2O 2H2O2![]() 2H2O + O2��
2H2O + O2��
��������
(1)�������Ǻ�������Ԫ�صĻ��������һ��Ԫ������Ԫ�أ�����H2O2��
(2)��ѧ��Ӧǰ�����ԭ�Ӹ�����ȣ���Ӧǰ�����ԭ�Ӹ����IJ�����X������ԭ�ӵĸ���������X�Ļ�ѧʽ��Cl2������������к���ϡ���ᣬ����ʯ�������к���̼��ƣ�̼�����ϡ���ᷴӦ�����Ȼ��ơ�ˮ��������̼��
(3)����������ǿ�ҵĸ�ʴ�ԣ��ܸ�ʴ�˵�Ƥ�����������������Ч�ɷ���ϡ���ᣬ���������Ʒ�Ӧ�����Ȼ��ƺ�ˮ��
(4)����Ư��������Ч�ɷ��ǹ������⣬��ʹ��ʱ���ͷų�����ʹ���ձ��������ϴ�ӣ��䷴Ӧԭ����2H2O![]() 2H2O + O2��
2H2O + O2��

 ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д� ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�����Ŀ��������ҹ���������ʳ����ʷ�ƾá�������õ�һ��ȼ���ǹ���ƾ���ij��ѧ��ȤС���ͬѧ��������ƾ��������˺��棬����ɷֽ���̽��������ش��������⡣
���������ϣ�
a������ƾ�Ҳ����Ϊ"�ƾ���"�����ȼ�Ͽ顣����ƾ������ǹ���״̬�ľƾ����ǽ��ƾ���Ӳ֬����������ư�һ���������Ȼ���Ƴɡ�
b���ƾ��Ļ�ѧʽΪC2H5OH��
c.�Ȼ������Ȼ�����Һ�������ԡ�
d. BaCl2+Na2CO3=BaCO3��+2NaCl ���ɵ�BaCO3Ϊ��ɫ����
��������⣩
(1)�ƾ��Ļ�ѧʽ��NaOH��ȣ�������OH������ô�ƾ���ˮ��Һ�Dz����Լ��ԣ�
(2)����ƾ��е����������Ƿ���ʼ����ʵij̶���Σ�
��ʵ��̽��1���ƾ���ˮ��Һ�Dz����Լ���
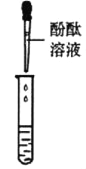
ͬѧ��ȡ�����ƾ���Һ���Թ��У��μ���ɫʯ����Һ��δ�۲쵽��ɫʯ���Ϊ��ɫ��˵���ƾ���Һ_______(������������������)���ԡ�
��ʵ��̽��2������ƾ��е����������Ƿ���ʼ����ʵij̶����
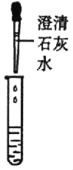
�ٹ���ƾ��е����������Ƿ���ʣ�ͬѧ����ȡ��������ƾ����ձ��У���������ˮ�ܽ��μ�������ϡ���ᣬ�۲쵽__________����˵�����������ѱ��ʣ���д�����������ڿ����б��ʵĻ�ѧ����ʽ______________��
��Ϊ��һ��ȷ���������Ƶı��ʳ̶ȣ��������̽����
����ͬѧȡ�ձ��ϲ���Һ����֧�Թ��У�����ͼ��ʾ����ʵ�顣
ʵ�鷽�� |
|
|
ʵ������ | ��Һ��� | ����__ |
ʵ����� | ��Һ������������ | ��Һ����̼���� |
����ͬѧ��Ϊ����ʵ�鲻��֤����Һ��һ�����������ƣ�������__��������ȡ�ձ����ϲ���Һ���������Ȼ�����Һ����ַ�Ӧ���ã�ȡ�ϲ���Һ���μӷ�̪��Һ����̪��Һ��졣
����˼����������ʵ���м������Ȼ�����Һ��Ŀ����________��
��ʵ�����]С��ͬѧ�������ۣ�һ����Ϊ�ù���ƾ��е��������Ʋ��ֱ��ʡ�