��Ŀ����
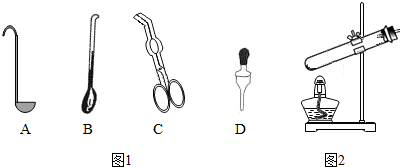
17����ͼ1��ľ̿��������ȼ��ʵ��IJ���ʾ��ͼ����ش��������⣺
��1��ȡ��ľ̿������������ǯ��
��2���Ѻ��ȵ�ľ̿����ʢ�������ļ���ƿʱ��Ӧ���������»������룬������ľ̿��������ַ�Ӧ��
��4����ͼ2���������������ȼ�յķ�Ӧ���ش��������⣺
�٣�������������ƿ�ײ���ˮ����ʲô���ã�
�ף���ֹ������ۻ���ը��ƿ�ף�
�ң����ն����������壬��ֹ��Ⱦ������
�ڼ���ƿ�ײ���ˮ�ܷ���ϸɳ��Ϊʲô����ƿ�еײ���ˮ�ܻ���ϸɳ��ϸɳ�ܽ�ƿ������ۻ����������ƿ�еײ���ˮ���ܻ���ϸɳ��ϸɳ�������ն����������壮
���� ��1������ʵ�����г��������������ơ���;�Լ�ȼ�յ�����������գ�
��2���Ѻ��ȵ�ľ̿��ƿ�����»����������ʹľ̿��������ַ�Ӧ���ݴ˷����ش�
��3��������������������ȼ�յ�ע����������ش�
��� �⣺��1����ͼʾ��֪��ȡ��ľ̿������������ǯ��
��2������Ѻ��ȵ�ľ̿�ܿ�ز���ʢ�����ļ���ƿ�²���ȼ�����ɵ��ȺͶ�����̼�Ὣ���ϲ���δ��Ӧ�������ų���ʹȼ�ղ��ܼ������У�Ϊʹľ̿��������ַ�Ӧ����ȷ�IJ��������ǰѼ��������ȵ�ľ̿��ƿ�����»������룮
��3���ټ�ƿ������������ȼ�գ���ˮ�������ǣ���ֹ������ۻ���ը��ƿ�ף���ƿ������������ȼ�������˶����������壬����Ⱦ���������Լ�ˮ�������ǣ����ն����������壬��ֹ��Ⱦ������
�ڼ�ƿ�еײ���ˮ�ܻ���ϸɳ��ϸɳ�ܽ�ƿ������ۻ����������ƿ�еײ���ˮ���ܻ���ϸɳ��ϸɳ�������ն����������壮
�ʴ�Ϊ����1������ǯ��
��2��ľ̿��������ַ�Ӧ��
��3���ٷ�ֹ������ۻ���ը��ƿ�ף��ڼ�ƿ�еײ���ˮ�ܻ���ϸɳ��ϸɳ�ܽ�ƿ������ۻ����������ƿ�еײ���ˮ���ܻ���ϸɳ��ϸɳ�������ն����������壮
���� ���⿼�����������ʵ�ʵ�飬���ڻ����ԵĿ��飬�ѶȲ��ؼ�����ʵ�������������ã�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� |  ��˿��������ȼ�� | B�� |  �㵹Һ�� | ||
| C�� |  ��ȡҺ������ | D�� |  ��ȡ����ˮ |
| A�� | �߲˸��� | B�� | ������ | C�� | ����ͨ����� | D�� | ����ĺ��� |
��1��ͬѧ����̽��˫��ˮ���������������Է�Ӧ���ʵ�Ӱ�죬������������ͬ������½���������ʵ�飬��¼���ռ�һƿ��ͬ�����������Ҫ��ʱ�䣮
| ʵ�� | 30%˫��ˮ��������g�� | �����ˮ�������mL�� | ��������������g�� | �ռ�ʱ�䣨s�� |
| 1 | 10 | 40 | 5 | 200 |
| 2 | 20 | 30 | 5 | 100 |
| 3 | 30 | 20 | 5 | 67 |
��д��������Ӧ�Ļ�ѧ��Ӧ����ʽ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��ʵ��3�У�����Ķ�����������Ϊ5g��
����ͬ�����£�ʵ��3����������������죬˵����ͬ�����£���Ӧ��Ũ��Խ��Ӧ����Խ�죮
��2��ͬѧͨ���������ϵ�֪��˫��ˮ��70�����ϻ�Ͽ�ֽ����������Ϊ����֤����˫��ˮҲ���Բ���������ͬѧ��ѡ��ͼ2װ�ã����������ã�����ʵ�飮ʵ���й۲쵽�Թ��ڲ������ݣ����������ǵ�ľ�����ڵ��ܿ�û�и�ȼ�����ܵ�ԭ���������л��н϶��ˮ������ͬѧ����ԭ���������ˮ���ռ����壬�ټ��飬֤������˫��ˮҲ�ɲ���������
����ʦ���ѡ�����Cu��Fe2O3���ֹ����е�һ�ֻ��������
��������롿��ɫ��ĩ���ܵ���ɣ�
�����ֻ��Cu�� �����ֻ��Fe2O3�� �������Cu��Fe2O3�Ļ����
��ʵ��̽����
ͬѧ��Ϊȷ����ɫ��ĩ����ɣ���ȡ�÷�ĩ5.0gװ��Ӳ�ʲ������У���ͼ1��ͨ����н���ʵ�飮��ʼʱ����ͨ��CO���壬��һ��ʱ����ټ���ʹ���ַ�Ӧ������Ӧ��ȫ��ֹͣ���ȣ�����ͨCO����ֱ����������ȴ��

��1����װ��A����ͨCO�����Ŀ�����ž�װ���еĿ�������ֹ����ʱ������ը��Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��
��2����Ӧǰ��������װ�ú����ʵ������������������±���
| ��Ӧǰ | ��Ӧ�� |
| �����ܺͺ�ɫ��ĩ��������Ϊ37.3g | �����ܺ������ʵ�������Ϊ36.1g |
��3��С��ͬѧ��ΪΪ�˷�ֹ��Һ��������ʵ��ֹͣ����ǰӦ�ȶϿ�A��B�����ӣ�С����Ϊ����Ҫ����������Ϊһֱͨ��CO���壬B����Һ���ᵹ����Aװ���У�
����˼���ۡ�ʵ���С������ͬѧָ������ͼװ��δ����β���Ĵ�����������ͬѧ�������ͼ2װ�ô���ԭװ�ã�ʵ��װ��ͼ�в������߿��ڵ�װ�ã���������AB��
A���ռ�һ����̼ B�����ն�����̼ C�����������̼��
| ���� | ϴ�ྫ | ����� | ¯������ | ���ձ�ը�� | Ư�� |
| ��Ʒ��ʽ |  |  |  |  |  |
| ��Ч�ɷֻ��� | ��ϴ���� | ���� | �������� | ��̼���� | ���� |
��2���������ʿ���ʹ�ý������ϴ����ac������ĸ��ţ���
a�����⡡����������b�����ա���������c��ˮ��
��3�������������¯��������ϣ����Է�����ͼ1��ʾ�Ļ�ѧ��Ӧ��ͼ��a���Ļ�ѧʽΪH2O���÷�Ӧ�Ļ�������Ϊ���ֽⷴӦ
��4���ں�ۡ��ۡ�����֮�佨����ϵ���ǻ�ѧѧ�����е�˼ά��ʽ����ͼ��ʾ��HCl��ˮ�л�����H+��Cl-��NaOH��ˮ�л���봦Na+��OH-���Դ�����H2SO4��ˮ�л�������������H+��SO42-�������ӷ��ţ�
��5��������ͼ2�е�ʵ���������Թܡ��ձ����ƾ��ơ����������¶ȼơ�PH��ֽ�ȣ���ҩƷ����ɫʯ����Һ����ɫ��̪��Һ��ϡ���ᡢ����������Һ�ȣ���������ֲ�ͬ�ķ���֤����ͼ2�е��������ʷ����˻�ѧ��Ӧ
����һ����ʢ������������Һ���Թ��еμӷ�̪��Һ����Һ��죬Ȼ����εμ�ϡ���ᣬ������ɫ
���������ֱ���pH��ֽ��ϡ���������������Һ��pH��Ȼ���Ϻ��ٲ���Һ��pH
��6����ҵ�Ͻ�������Cl2��ͨ���ռ���Һ�п���ȡ����Һ����Ӧ���γ���NaCl��NaClO����Һ���÷�Ӧ�Ļ�ѧ����ʽΪCl2+2NaOH=NaCl+NaClO+H2O

