��Ŀ����
ij̼������Ʒ�к��������Ȼ������ʣ�Ϊ�ⶨ����Ʒ��̼���Ƶ���������������������ʵ�飺

��ʵ��һ��
��ش��������⣺
������A���õ�����������������________��
����ͬѧȡ������̼������Ʒ����Һ��������ɫ��̪��Һ����Һ����ɫ���________��
������ʵ������м��뱥��ʯ��ˮ������Ӧ�Ļ�ѧ����ʽ��________��
������Ϊ̽��������Ӧ����Һ�е����ʳɷ֣���ͬѧ����Һ�еμӹ���ϡ���ᣬ����
�����ݲ�������μ�����ǰ��Һ�е����ʳ��Ȼ������________��
��ʵ�����
��ͬѧȡ12g��̼������Ʒ�����ձ��У�����100gϡ���ᣨ����������ȫ��Ӧ����
����Һ����Ϊ107.6g���Լ��㣺
�������ɶ�����̼���ʵ���Ϊ________mol��
����̼������Ʒ��Na2CO3��������������д��������̣�������0.1%��________��
���������õĽ����ʵ�ʵ����������ߣ����ܵ�ԭ����________������һ�ּ��ɣ�
���� ��ɫ Na2CO3+Ca��OH��2�TCaCO3��+2NaOH ̼���ơ��������� 0.1 88.3% ϡ����ӷ�����HCl������CO2�����ų����Ӷ�����������Ʒ��̼���Ƶ���������ƫ��
�������٣����ݹ����в����������÷�����
��������ɫ��̪��������з�����
����̼���ƺ��������Ʒ�Ӧ����̼��Ƴ������������ƣ�����Ϸ���ʽ����д������д����ʽ��
���������ݷ�Ӧ����ͼ������Һ����̼���ƺ��������Ʒ�Ӧ���ɵ��������ƣ��μӹ���ϡ���ᣬ���������ݲ�����˵��̼������Һ����������Һ�л�����̼���ƣ�
�ڣ����������غ㶨�ɷ�����
�����ݻ�ѧ����ʽNa2CO3+2HCl�T2NaCl+H2O+CO2�������ɶ�����̼���������Լ����̼������Ʒ��Na2CO3���������Ӷ������̼���Ƶ�����������
����ϡ����ӷ�����HCl�����ˮ������CO2�����ų����Ӷ�����������Ʒ��̼���Ƶ���������ƫ��
��𣺢٣�����A�ǹ��ˣ����в��������������������ʴ�Ϊ��������
����̼������Һ�Լ��ԣ�������ɫ��̪��Һ����Һ����ɫ��ɺ�ɫ���ʴ�Ϊ����ɫ��
������Ʒ�е��Ȼ������������Ʋ���Ӧ��̼���ƺ��������Ʒ�Ӧ����̼��Ƴ������������ƣ�����ʽ��Na2CO3+Ca��OH��2�TCaCO3��+2NaOH��
�ʴ�Ϊ��Na2CO3+Ca��OH��2�TCaCO3��+2NaOH��
��������Һ����̼���ƺ��������Ʒ�Ӧ���ɵ��������ƣ�����μӹ���ϡ���ᣬ���������ݲ�����˵��̼������Һ���������Ը���Һ�л�����̼���ƣ�
�ʴ�Ϊ��̼���ơ��������ƣ�
�ڣ����������غ㶨�ɿ�֪�����ɶ�����̼���ʵ�����Ϊ12g+100g-107.6g=4.4g��������̼��Ħ��������44g/mol��������ɶ�����̼���ʵ���Ϊ =0.1mol���ʴ�Ϊ��0.1��
=0.1mol���ʴ�Ϊ��0.1��
������̼������Ʒ��Na2CO3������������Ϊx����
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 4.4g
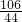 =
=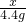
���x=10.6g
����Ʒ��̼���Ƶ���������Ϊ��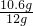 ��100%=88.3%��
��100%=88.3%��
�ʴ�Ϊ��88.3%��
���� ϡ����ӷ�����HCl������CO2�����ų����Ӷ�����������Ʒ��̼���Ƶ���������ƫ�ʴ�Ϊ��ϡ����ӷ�����HCl������CO2�����ų����Ӷ�����������Ʒ��̼���Ƶ���������ƫ��
���������⿼����ѧ�������ͽ���������������ȷÿһ�������ú��������Ļ�ѧ��Ӧ��ͬʱҪ�������Ǹ��ݻ�ѧ��Ӧ����ʽ�ļ��㲽�衢��ʽ�Լ���֮��ص�֪ʶ�ȣ��ѶȲ���
�������٣����ݹ����в����������÷�����
��������ɫ��̪��������з�����
����̼���ƺ��������Ʒ�Ӧ����̼��Ƴ������������ƣ�����Ϸ���ʽ����д������д����ʽ��
���������ݷ�Ӧ����ͼ������Һ����̼���ƺ��������Ʒ�Ӧ���ɵ��������ƣ��μӹ���ϡ���ᣬ���������ݲ�����˵��̼������Һ����������Һ�л�����̼���ƣ�
�ڣ����������غ㶨�ɷ�����
�����ݻ�ѧ����ʽNa2CO3+2HCl�T2NaCl+H2O+CO2�������ɶ�����̼���������Լ����̼������Ʒ��Na2CO3���������Ӷ������̼���Ƶ�����������
����ϡ����ӷ�����HCl�����ˮ������CO2�����ų����Ӷ�����������Ʒ��̼���Ƶ���������ƫ��
��𣺢٣�����A�ǹ��ˣ����в��������������������ʴ�Ϊ��������
����̼������Һ�Լ��ԣ�������ɫ��̪��Һ����Һ����ɫ��ɺ�ɫ���ʴ�Ϊ����ɫ��
������Ʒ�е��Ȼ������������Ʋ���Ӧ��̼���ƺ��������Ʒ�Ӧ����̼��Ƴ������������ƣ�����ʽ��Na2CO3+Ca��OH��2�TCaCO3��+2NaOH��
�ʴ�Ϊ��Na2CO3+Ca��OH��2�TCaCO3��+2NaOH��
��������Һ����̼���ƺ��������Ʒ�Ӧ���ɵ��������ƣ�����μӹ���ϡ���ᣬ���������ݲ�����˵��̼������Һ���������Ը���Һ�л�����̼���ƣ�
�ʴ�Ϊ��̼���ơ��������ƣ�
�ڣ����������غ㶨�ɿ�֪�����ɶ�����̼���ʵ�����Ϊ12g+100g-107.6g=4.4g��������̼��Ħ��������44g/mol��������ɶ�����̼���ʵ���Ϊ
 =0.1mol���ʴ�Ϊ��0.1��
=0.1mol���ʴ�Ϊ��0.1��������̼������Ʒ��Na2CO3������������Ϊx����
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 4.4g
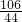 =
=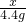
���x=10.6g
����Ʒ��̼���Ƶ���������Ϊ��
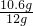 ��100%=88.3%��
��100%=88.3%���ʴ�Ϊ��88.3%��
���� ϡ����ӷ�����HCl������CO2�����ų����Ӷ�����������Ʒ��̼���Ƶ���������ƫ�ʴ�Ϊ��ϡ����ӷ�����HCl������CO2�����ų����Ӷ�����������Ʒ��̼���Ƶ���������ƫ��
���������⿼����ѧ�������ͽ���������������ȷÿһ�������ú��������Ļ�ѧ��Ӧ��ͬʱҪ�������Ǹ��ݻ�ѧ��Ӧ����ʽ�ļ��㲽�衢��ʽ�Լ���֮��ص�֪ʶ�ȣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
Ϊ��֤����ȥʳ�ξ����к���������Na2CO3��Na2SO4�������ʣ������������ʵ�鷽����

��ش�
��1��ʳ�ξ����м���A��Һ��______������1������______�������л������룮
��2����Һ���к��е�������______������2��______���ʣ�
��3�����ô�ʵ�鷽�����õ�NaCl������������Ʒ��NaCl��������______������ࡱ���١���
��4��Ϊ�˲ⶨ50�˵��������ƺ�̼���ƻ����Һ���������Ƶĺ�����ijʵ��̽��С�������кͷ�Ӧ��ԭ������ʵ�飬ʵ�鲽�����£�
���裨һ������ȥ���Һ�е�̼����
| ʵ�鲽�� | ʵ������ |
| �ڻ��Һ�м���������Ȼ�����Һ����ַ�Ӧ����ˣ��õ�������Һ | ������ɫ���� |
| ������Һ�е������ǣ�______�ѧʽ�� | |
 ˮ����Ҫ����Դ�����˼�һ����������������ģ���Ȼ���ˮ���и������ʣ�������ֱ��ʹ�ã�����о�����
ˮ����Ҫ����Դ�����˼�һ����������������ģ���Ȼ���ˮ���и������ʣ�������ֱ��ʹ�ã�����о����� ?
? ˮ����Ҫ����Դ�����˼�һ����������������ģ���Ȼ���ˮ���и������ʣ�������ֱ��ʹ�ã�����о�����
ˮ����Ҫ����Դ�����˼�һ����������������ģ���Ȼ���ˮ���и������ʣ�������ֱ��ʹ�ã�����о�����