��Ŀ����
��6�֣��������������������й㷺��Ӧ�á�
��1�����н�����Ʒ�У����ý��������Ե���_________������ĸ��ţ���
A���ƽ����� B��������
B�������� C���ֵ���
C���ֵ���
��2������Ʒ��ɳĮ����������ʴ��ԭ����___________��
��3����ҵ����һ����̼�ͳ�����������Ӧ�Ļ�ѧ����ʽΪ____________��
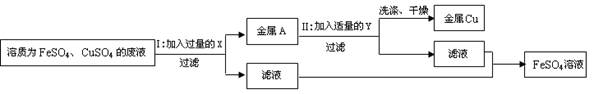
��4��ij���ŷŵķ�ˮ�к�������ͭ������ˮ�����ɵõ�ͭ�����죨�����������ֲ�Ʒ����������ͼ��ʾ����ˮ�е��������ʲ����뷴Ӧ����
���̢��з�����Ӧ�Ļ�ѧ����ʽΪ_________��
A-G���������к���Ԫ�ص���___________������ĸ���ţ���
��1�����н�����Ʒ�У����ý��������Ե���_________������ĸ��ţ���
A���ƽ�����
 B��������
B�������� C���ֵ���
C���ֵ���
��2������Ʒ��ɳĮ����������ʴ��ԭ����___________��
��3����ҵ����һ����̼�ͳ�����������Ӧ�Ļ�ѧ����ʽΪ____________��
��4��ij���ŷŵķ�ˮ�к�������ͭ������ˮ�����ɵõ�ͭ�����죨�����������ֲ�Ʒ����������ͼ��ʾ����ˮ�е��������ʲ����뷴Ӧ����

���̢��з�����Ӧ�Ļ�ѧ����ʽΪ_________��
A-G���������к���Ԫ�ص���___________������ĸ���ţ���
(1)C������2��û��ˮ�֡���
��3��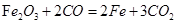
��4��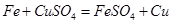 �� A��B��C��E��G
�� A��B��C��E��G
��3��
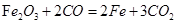
��4��
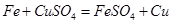 �� A��B��C��E��G
�� A��B��C��E��G��1��A���ƽ���Ʒ�����ý�������չ�ԣ� B�������������ý����ĵ����ԣ� C��ͭ�������ý����ĵ����ԡ�
��2������ʴ��������������е�ˮ�������Ӵ�����������Ʒ��ɳĮ����������ʴ��ԭ���ǿ����������ˮ�֡�
��3�����������Ҫ�ɷ�����������һ����̼��ԭ�������Ļ�ѧ����ʽ�ǣ�3CO+Fe2O3 2Fe+3CO2��
2Fe+3CO2��
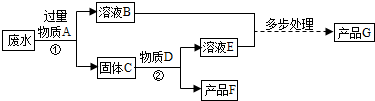
��4������A�ǹ�������������A���� Fe+CuSO4�TFeSO4+Cu�����ù���C������ͭ�Ļ�����ҺB��������������Һ������C������D��D��ϡ���ᣬ������Ӧ��ͭ����Ӧ�Ӷ�ʹ��������ȫ��ת��Ϊ��Ʒ����ҺD����������ҺE������������ϡ����Ļ�����ƷG�������졣
��2������ʴ��������������е�ˮ�������Ӵ�����������Ʒ��ɳĮ����������ʴ��ԭ���ǿ����������ˮ�֡�
��3�����������Ҫ�ɷ�����������һ����̼��ԭ�������Ļ�ѧ����ʽ�ǣ�3CO+Fe2O3
 2Fe+3CO2��
2Fe+3CO2����4������A�ǹ�������������A���� Fe+CuSO4�TFeSO4+Cu�����ù���C������ͭ�Ļ�����ҺB��������������Һ������C������D��D��ϡ���ᣬ������Ӧ��ͭ����Ӧ�Ӷ�ʹ��������ȫ��ת��Ϊ��Ʒ����ҺD����������ҺE������������ϡ����Ļ�����ƷG�������졣

��ϰ��ϵ�д�
�����Ŀ