��Ŀ����
(2008������)����������װ��ͼ�ش����⣺
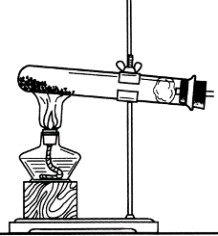
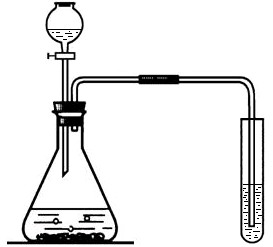
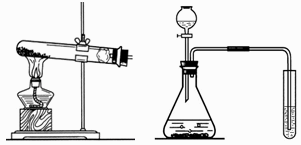
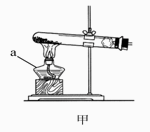
(1)д���б�����������ƣ�a____________��b____________��
(2)ʵ�����ü�װ���������Ļ�ѧ����ʽ��______________________________����____________���ռ���������Ӧ��������ȴ�����Թ��м���������ˮ�����衢���ˣ��õ���ɫ��ĩ���ú�ɫ��ĩ���������Ӵ��д������ݲ�������Ӧ�Ļ�ѧ����ʽ��____________________________________����ɫ��ĩ�ڷ�Ӧ�е�������____________��
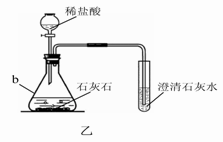
(3)ij��ѧ��ȤС������װ����ȡ�����������̼��ʵ������У��ɹ۲쵽�Թ������____________ɫ��������Ӧ�Ļ�ѧ����ʽ��____________________________________���������������ͨ�������̼����һ��ʱ����ֳ����ܽ��ɳ�����Һ��Ϊ��ȷ�������ܽ�ɳ�����Һ��ԭ��С���ͬѧ���������̽����
������� ����Ϊʲô���ܽ��ɳ�����Һ��
�������� ̼���������ᣬ̼����ƣ�Ca(HCO3)2������ˮ��
��������� ����Һ�����ԣ��ڷ�Ӧ������̼����ơ�
ʵ�������
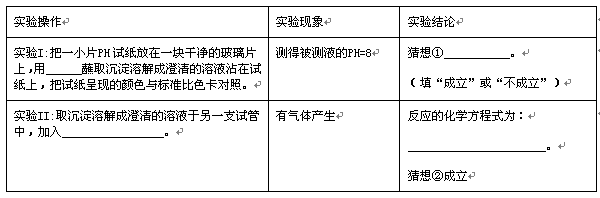
ʵ����� | ʵ������ | ʵ����� |
ʵ���һСƬpH��ֽ����һ��ɾ��IJ���Ƭ�ϣ���_________պȡ�����ܽ�ɳ������Һմ����ֽ�ϣ�����ֽ���ֵ���ɫ�����ɫ�����ա� | ��ñ���Һ��pH��8 | �����______________�� (���������������) |
ʵ���ȡ�����ܽ�ɳ������Һ����һ֧�Թ��У�����______________________ ___________________�� | ��������� | ��Ӧ�Ļ�ѧ����ʽΪ�� ______________________________________�� ����ڳ����� |
ͨ��̽����֪�����ɵij������������̼��ˮ��Ӧ�����˿�����ˮ��̼����ơ������뷴˼��̽������õ�����ʾ�������___________________________��
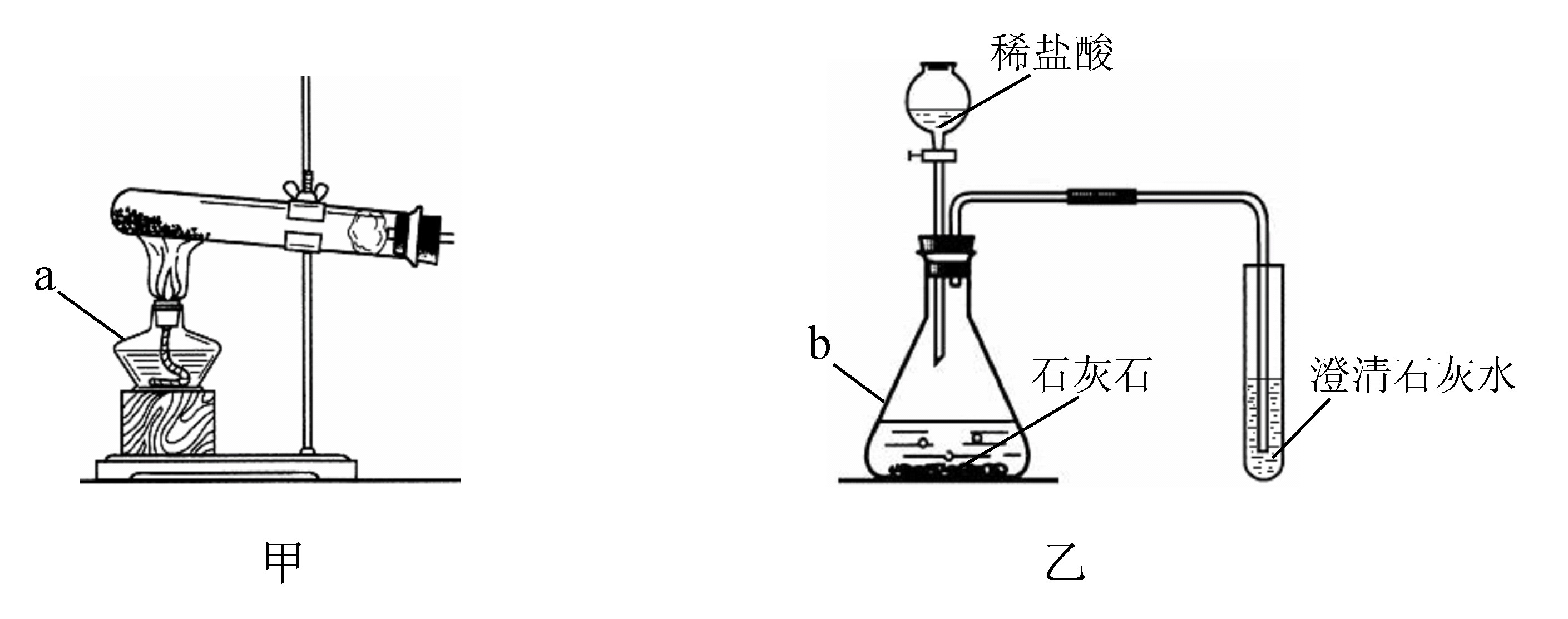
(1)a.�ƾ��� b.��ƿ (2)2KMnO4 �� KMnO4 + MnO2 + O2�� ��ˮ��(�������ſ�����) 2H2O2 === 2H2O + O2�� ������ (3)�� CO2 + Ca(OH)2 = CaCO3��+ H2O ʵ������� ʵ������� ������ ʵ���ϡ���� 2HCl + Ca(HCO3)2 = CaCl2 + 2H2O + 2CO2�� �����뷴˼����������һ�������¿�ת��Ϊ������(�������������)

 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�