��Ŀ����
���������Ũ���Ṳ�Ȼ�ֽ⣺H2C2O4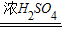 CO��+CO2��+H2O��
CO��+CO2��+H2O����ѧ��ȤС�齫����ֽ�����Ļ����������ͨ������װ�ã�Ŀ���ǣ��ټ������ֽ��������CO2��H2O�����ô�����CO��ԭFe2O3���������������Ʒ��Fe2O3��������������˵�����������е����ʲ���Ӧ������ÿ���ķ�Ӧ�����ն�����ȫ�ģ�
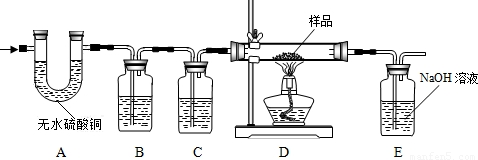
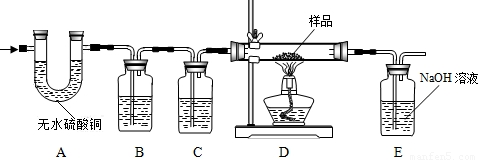
��1��A�е���ˮ����ͭ��ˮ������������������ˮ�Ĵ��ڣ�
��2��B�������� ������װ���� ��Һ��C��װ��Ӧ���� ������������ ��
��3��ʵ��ʱ��A��B��λ�ò��ܽ������������������ ��
��4��װ��E�з�����Ӧ�Ļ�ѧ����ʽΪ ������ȤС���ͬѧΪ����ɡ�Ŀ�Ģڡ���ֻ��������Eװ���ڷ�Ӧǰ������������û��ʵ�֣�ԭ���� ��
��5���ӶԻ���Ӱ��ĽǶȿ��ǣ�����Ʋ�����֮���� ��
���𰸡���������2�����������̼�ó���ʯ��ˮ������һ����̼����Ũ���
��3���������Һ��ͨ��ʱ�����ˮ������Ӱ��ʵ������
��4�����ݷ�Ӧ�����������д��ѧ����ʽ����ʱ��������Ʒ��Fe2O3����������Ҫ֪�����������������������������
��5��һ����̼�ж�������ֱ���ŷŵ������У�
����⣺��2�����������̼Ҫ�ó���ʯ��ˮ����B����װ���� ����ʯ��ˮ��Һ��ͨ�벣�����е�һ����̼Ӧ���Ǹ���ģ���C��װ��Ӧ����Ũ���ᣬ���������Ǹ���һ����̼��
��3�������������Ļ����ʱ��Ҫע�ⲻ������ţ����ABװ�ý������Ͳ��ܼ������������Ƿ���ˮ����Ϊ�����ʯ��ˮ��ͨ��������ˮ������
��4��װ��E�з�����Ӧ�Ļ�ѧ����ʽΪ CO2+2NaOH=Na2CO3+H2O�����ֻ��������Eװ���ڷ�Ӧǰ����������Ͳ��ܲ����������Ʒ��Fe2O3������������ԭ���� û�г���Dװ���в����ܷ�Ӧǰ�����������֪������ʯ��������
��5���ӶԻ���Ӱ��ĽǶȿ��ǣ�����Ʋ�����֮����β��û�д�����һ����̼����ֱ���ŷŵ������У�����Ⱦ������
�ʴ�Ϊ����2�����������̼������ʯ��ˮ��Ũ���ᣬ����һ����̼��
��3�����ܼ������������Ƿ���ˮ��
��4��CO2+2NaOH=Na2CO3+H2O��û�г���Dװ���в����ܷ�Ӧǰ���������
��5��β��û�д�����
����������ʵ��Ŀ�ļ�����Ƶ�ʵ��װ�ã���ȷÿ��װ����ʵ������������ã��ǽ��ô�����Ļ�����ؼ��������ѶȽϴ��ۺ��Խ�ǿ��
��3���������Һ��ͨ��ʱ�����ˮ������Ӱ��ʵ������
��4�����ݷ�Ӧ�����������д��ѧ����ʽ����ʱ��������Ʒ��Fe2O3����������Ҫ֪�����������������������������
��5��һ����̼�ж�������ֱ���ŷŵ������У�
����⣺��2�����������̼Ҫ�ó���ʯ��ˮ����B����װ���� ����ʯ��ˮ��Һ��ͨ�벣�����е�һ����̼Ӧ���Ǹ���ģ���C��װ��Ӧ����Ũ���ᣬ���������Ǹ���һ����̼��
��3�������������Ļ����ʱ��Ҫע�ⲻ������ţ����ABװ�ý������Ͳ��ܼ������������Ƿ���ˮ����Ϊ�����ʯ��ˮ��ͨ��������ˮ������
��4��װ��E�з�����Ӧ�Ļ�ѧ����ʽΪ CO2+2NaOH=Na2CO3+H2O�����ֻ��������Eװ���ڷ�Ӧǰ����������Ͳ��ܲ����������Ʒ��Fe2O3������������ԭ���� û�г���Dװ���в����ܷ�Ӧǰ�����������֪������ʯ��������
��5���ӶԻ���Ӱ��ĽǶȿ��ǣ�����Ʋ�����֮����β��û�д�����һ����̼����ֱ���ŷŵ������У�����Ⱦ������
�ʴ�Ϊ����2�����������̼������ʯ��ˮ��Ũ���ᣬ����һ����̼��
��3�����ܼ������������Ƿ���ˮ��
��4��CO2+2NaOH=Na2CO3+H2O��û�г���Dװ���в����ܷ�Ӧǰ���������
��5��β��û�д�����
����������ʵ��Ŀ�ļ�����Ƶ�ʵ��װ�ã���ȷÿ��װ����ʵ������������ã��ǽ��ô�����Ļ�����ؼ��������ѶȽϴ��ۺ��Խ�ǿ��

��ϰ��ϵ�д�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
�����Ŀ
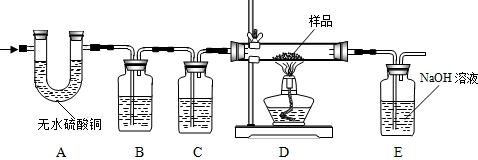
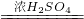 CO��+CO2��+H2O��
CO��+CO2��+H2O��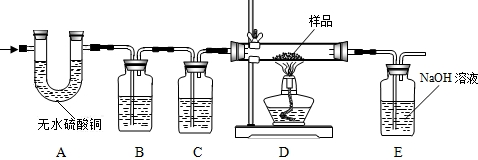
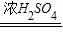 CO��+CO2��+H2O��
CO��+CO2��+H2O��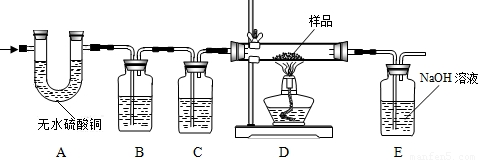
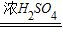 CO��+CO2��+H2O��
CO��+CO2��+H2O��