��Ŀ����
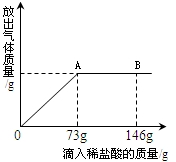 ��֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��43.1g Na2CO3��Һ���������μ������ʷ���Ϊ10%��ϡ���ᣮNa2CO3+2HCl�T2NaCl+CO2��+H2O���ų������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��43.1g Na2CO3��Һ���������μ������ʷ���Ϊ10%��ϡ���ᣮNa2CO3+2HCl�T2NaCl+CO2��+H2O���ų������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺��1�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��pH
��2�����μ�ϡ������ͼ��A��ʱ���ձ���Ϊ��������Һ�����£���ͨ����������������ʵ��������������������С�����һλ���֣�
��3����֪������NaCl���ܽ��Ϊ36g���μ�ϡ������ͼ��A��ʱ����Ҫʹ��ʱ����Һ��Ϊ������Һ�����£���ͨ�����㻹�����������������ʣ����������С�����һλ���֣���
��������1����ͼ��֪����B��ʱ�������Ѿ������������Һ��ʱ�����ԣ�pHС��7��
��2����ϡ����������������������ݻ�ѧ����ʽ���Լ���������Ȼ��ƺͶ�����̼�����������������������Һ�����ʵ�����������
��3�����ñ�����Һ�����ʺ��ܼ��������ȿ��Լ������Ҫ�����Ȼ��Ƶ�������
��2����ϡ����������������������ݻ�ѧ����ʽ���Լ���������Ȼ��ƺͶ�����̼�����������������������Һ�����ʵ�����������
��3�����ñ�����Һ�����ʺ��ܼ��������ȿ��Լ������Ҫ�����Ȼ��Ƶ�������
����⣺��1����ͼ��֪����B��ʱ�������Ѿ������������Һ��ʱ�����ԣ�pH��7��
��2���������Ȼ��Ƶ�����Ϊx�����ɶ�����̼������Ϊy��
Na2CO3+2HCl�T2NaCl+CO2��+H2O
73 117 44
73g��10% x y
=
=
��x=11.7g��y=4.4g
����A��ʱ������Һ�����ʵ���������Ϊ
��100%��10.5%��
��3������Ҫ�����Ȼ��Ƶ�����Ϊz��
36g��100g=��11.7g+z������43.1g+73g-4.4g-11.7g��
z=24.3g��
�𣺣�1������
��2������A��ʱ������Һ�����ʵ���������Ϊ10.5%��
��3������Ҫ����24.3g�Ȼ��ƣ�
��2���������Ȼ��Ƶ�����Ϊx�����ɶ�����̼������Ϊy��
Na2CO3+2HCl�T2NaCl+CO2��+H2O
73 117 44
73g��10% x y
| 73 |
| 73g��10% |
| 117 |
| x |
| 44 |
| y |
����A��ʱ������Һ�����ʵ���������Ϊ
| 11.7g |
| 43.1g+73g-4.4g |
��3������Ҫ�����Ȼ��Ƶ�����Ϊz��
36g��100g=��11.7g+z������43.1g+73g-4.4g-11.7g��
z=24.3g��
�𣺣�1������
��2������A��ʱ������Һ�����ʵ���������Ϊ10.5%��
��3������Ҫ����24.3g�Ȼ��ƣ�
������������Ҫ���麬�������ʵĻ�ѧ����ʽ������������������ļ��㣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮
����һ������pH��ֽ�ⶨNaOH��Һ��pH���ٵμ����ᣬ����������Һ��ͬʱ�ⶨ�����Һ��pH�������õ�pH��С��С��7����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
��1����pH��ֽ�ⶨ��Һ��pHʱ����ȷ�IJ����ǣ� ��
��2������ǿ������õ�pHС��7�������ɣ� ��
������������NaOH��Һ�еμӼ��η�̪��Һ����Һ�Ժ�ɫ��Ȼ���ٵμ����ᣬ�ɹ۲쵽��ɫ����ʧ����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
����ͬѧ����NaOH��Һ�еμӷ�̪��Һʱ��������һ��������������������Һ�е����̪��Һ����Һ����˺�ɫ������һ�����ɫ����ʧ�ˣ���С����������������ԭ���������²��룺�ٿ����Ƿ�̪��Һ������е�������Ӧ��ʹ��ɫ��ʧ���ڿ���������������Һ������еĶ�����̼��Ӧ��ʹ��ɫ��ʧ��
��1��Ϊ��֤����٣�����ͬѧ��������ʵ�飺�����Ƶ�����������Һ���ȣ�����Һ���Ϸ���һЩֲ���ͣ�Ȼ������ȴ�����Һ�е����̪��Һ��ʵ���С����ȡ��͡�����ֲ���͡�Ŀ���� ��ʵ����������̪��Һ��ɫ��ʧ������е������أ�
��2��Ϊ��֤����ڣ�����ͬѧ��������ʵ�飺ȡ��һ������Na2CO3��Һ�������е����̪��Һ��������ҺҲ���ֺ�ɫ���ɴ˿ɵó�����������ۣ�����1��˵��Na2CO3��Һ�� �ԣ�����2��˵����̪��Һ��ɫ��ʧ������еĶ�����̼�أ�
��3����С��ͬѧͨ���������ϵ�֪��������������ҺŨ�Ⱥܴ�ʱ���ͻ���������������������ʵ��֤���÷�����ȡ�õ�NaOH��ҺŨ�ȹ���ʵ�鷽�� ���ڹ۲쵽������ ��
����������ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ��
����ͬѧ����ͬŨ�ȵ������NaOH��Һ��10mL��ϣ�����
�ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t���������
��1������x= ��
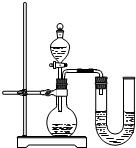
��2��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ���� �ж�NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ��
��3������ʵ���е�ϸ�ں������������ʵ���У�ϡ��������ý�ͷ�ι���εμӣ���������Ŀ���� ����ʵ������У�Ҫ�ò��������Ͻ��裬��������Ŀ���� ������ʵ����������ⷢ�������ݳ��֣�����Ϊԭ���� ���ܷ���ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ������������ѧ�Ļ�ѧ֪ʶ�������ְ�ɫ��ĩ�ijɷ��������²��룺�ٿ�����NaOH������Ϊ���ڿ����� �� �ۿ����� ��
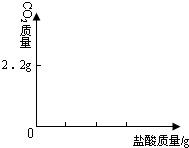
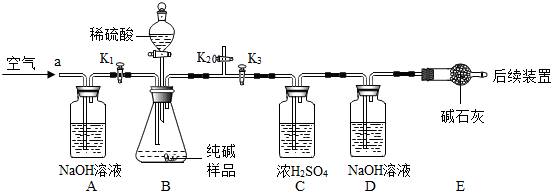
��4��Ϊ�˽�һ���о�ʵ���г��ֵ����⣬ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g10%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g����
��1����Ʒ���������Ƶ�������
��2�����������Ʒ�Ӧ�������������
��3����ͼ�л������������ʾ������̼�������������ʾ����������Ĺ�ϵͼ��
����֪Na2CO3+2HCl�T2NaCl+H2O+CO2����
����һ������pH��ֽ�ⶨNaOH��Һ��pH���ٵμ����ᣬ����������Һ��ͬʱ�ⶨ�����Һ��pH�������õ�pH��С��С��7����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
��1����pH��ֽ�ⶨ��Һ��pHʱ����ȷ�IJ����ǣ�
��2������ǿ������õ�pHС��7�������ɣ�
������������NaOH��Һ�еμӼ��η�̪��Һ����Һ�Ժ�ɫ��Ȼ���ٵμ����ᣬ�ɹ۲쵽��ɫ����ʧ����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
����ͬѧ����NaOH��Һ�еμӷ�̪��Һʱ��������һ��������������������Һ�е����̪��Һ����Һ����˺�ɫ������һ�����ɫ����ʧ�ˣ���С����������������ԭ���������²��룺�ٿ����Ƿ�̪��Һ������е�������Ӧ��ʹ��ɫ��ʧ���ڿ���������������Һ������еĶ�����̼��Ӧ��ʹ��ɫ��ʧ��
��1��Ϊ��֤����٣�����ͬѧ��������ʵ�飺�����Ƶ�����������Һ���ȣ�����Һ���Ϸ���һЩֲ���ͣ�Ȼ������ȴ�����Һ�е����̪��Һ��ʵ���С����ȡ��͡�����ֲ���͡�Ŀ����
��2��Ϊ��֤����ڣ�����ͬѧ��������ʵ�飺ȡ��һ������Na2CO3��Һ�������е����̪��Һ��������ҺҲ���ֺ�ɫ���ɴ˿ɵó�����������ۣ�����1��˵��Na2CO3��Һ��
��3����С��ͬѧͨ���������ϵ�֪��������������ҺŨ�Ⱥܴ�ʱ���ͻ���������������������ʵ��֤���÷�����ȡ�õ�NaOH��ҺŨ�ȹ���ʵ�鷽��
����������ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ��
����ͬѧ����ͬŨ�ȵ������NaOH��Һ��10mL��ϣ�����
�ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t���������
| ��� | ���� | NaOH��Һ | ��t/�� |
| 1 | 3.65% | 2.00% | 3.5 |
| 2 | 3.65% | 4.00% | x |
| 3 | 7.30% | 8.00% | 14 |

��1������x=
��2��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ����
��3������ʵ���е�ϸ�ں������������ʵ���У�ϡ��������ý�ͷ�ι���εμӣ���������Ŀ����
��4��Ϊ�˽�һ���о�ʵ���г��ֵ����⣬ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g10%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g����
��1����Ʒ���������Ƶ�������
��2�����������Ʒ�Ӧ�������������
��3����ͼ�л������������ʾ������̼�������������ʾ����������Ĺ�ϵͼ��

����֪Na2CO3+2HCl�T2NaCl+H2O+CO2����
 �ᡢ������й㷺��;����Ҫ�����ij��ѧ�С���ͬѧΧ���⼸����������һϵ�е�̽�����
�ᡢ������й㷺��;����Ҫ�����ij��ѧ�С���ͬѧΧ���⼸����������һϵ�е�̽�����
 ��2011?�����ʼ죩���������ƹ����п��ܻ���̼���ƣ��ס��ҡ�����λͬѧ�ֱ�ȡ������ˮ���м��飮
��2011?�����ʼ죩���������ƹ����п��ܻ���̼���ƣ��ס��ҡ�����λͬѧ�ֱ�ȡ������ˮ���м��飮