��Ŀ����
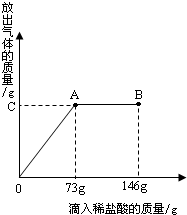 �á����������Ƽ���ƵõĴ�������������Ȼ��ƣ�Ϊ�ⶨij������Ʒ��̼���Ƶĺ�����С����ȡ�ô�����Ʒ12.4g���������μ������ʷ���Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ��������ͼ��ʾ�����������ش����⣺
�á����������Ƽ���ƵõĴ�������������Ȼ��ƣ�Ϊ�ⶨij������Ʒ��̼���Ƶĺ�����С����ȡ�ô�����Ʒ12.4g���������μ������ʷ���Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ��������ͼ��ʾ�����������ش����⣺��1�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��pH
��3����ô�����Ʒ��̼���Ƶ�����������������������һλС����
��������ͼ���֪������ϡ����73gʱ����Ʒ�е�̼���ƺ�ϡ����ǡ����ȫ��Ӧ����ʱ��Һ���Ȼ�����Һ�������ԣ�֮���ټ�ϡ������Һ�ͻ�����ԣ�Ȼ��ɸ���̼���ƺ�ϡ������ȫ��Ӧʱ���ĵ�����������������ɶ�����̼������������Ʒ��̼���Ƶ�������
����⣺��1���ɷ�����֪��B��ʱ���ӵ�ϡ�����Ѿ����������Դ�ʱ��Һ�����ԣ�
��2��������ϡ����73gʱ���ɵĶ�����̼����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
73 44
73g��10% x
=
��x=4.4g
����C���ֵΪ4.4
��3���贿����Ʒ��̼���Ƶ�����Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73
y 73g��10%
=
y=10.6g
���Դ�����Ʒ��̼���Ƶ���������Ϊ��
��100%=85.5%
�𣺣�1�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��pH��7��
��2����������C�����ֵΪ4.4��
��3���ô�����Ʒ��̼���Ƶ���������85.5%��
��2��������ϡ����73gʱ���ɵĶ�����̼����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
73 44
73g��10% x
| 73 |
| 44 |
| 73g��10% |
| x |
����C���ֵΪ4.4
��3���贿����Ʒ��̼���Ƶ�����Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73
y 73g��10%
| 106 |
| 73 |
| y |
| 73g��10% |
���Դ�����Ʒ��̼���Ƶ���������Ϊ��
| 10.6g |
| 12.4g |
�𣺣�1�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��pH��7��
��2����������C�����ֵΪ4.4��
��3���ô�����Ʒ��̼���Ƶ���������85.5%��
�����������Ǻ�ͼ�����ϵĻ�ѧ����ʽ�����⣬������Ĺؼ��Ƿ���ͼ�������ߵ���ֹ�����������ȡ���õ����ݽ��н��⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �á����������Ƽ���ƵõĴ�������������Ȼ��ƣ�Ϊ�ⶨij������Ʒ��̼���Ƶĺ�����С����ȡ�ô�����Ʒ2.4g������ܽ���ˮ�У��ٵμ��Ȼ�����Һ����������������������Ȼ�����Һ��������ϵ��ͼ��ʾ����
�á����������Ƽ���ƵõĴ�������������Ȼ��ƣ�Ϊ�ⶨij������Ʒ��̼���Ƶĺ�����С����ȡ�ô�����Ʒ2.4g������ܽ���ˮ�У��ٵμ��Ȼ�����Һ����������������������Ȼ�����Һ��������ϵ��ͼ��ʾ����