��Ŀ����
11�� ij��ѧ��ȤС��Ϊ�ⶨһ��ʯ��ʯ��Ʒ��̼��Ƶ�������������������ʵ�飺
ij��ѧ��ȤС��Ϊ�ⶨһ��ʯ��ʯ��Ʒ��̼��Ƶ�������������������ʵ�飺�ٳ�ȡ25.0gʯ��ʯ��Ʒ��ƽ���ֳ����ݣ����ֱ�ӹ��ɿ�״�ͷ�ĩ״��
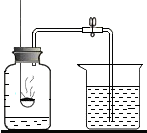
�ڽ�������Ʒ�ֱ�Ͷ��������ƿ�У���������������ͬ��������������ϡ���ᣨ��Ʒ�������ʲ�����ˮҲ�����ᷴӦ����������ɶ�����̼�������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
��ش�
��1��ÿ����Ʒ��ַ�Ӧ�����ɶ�����̼������Ϊ4.4g������Ʒ��̼��Ƶ���������Ϊ80%��
��2����ͼ�����߷����ó���Ӱ��û�ѧ��Ӧ���ʵ������Ƿ�Ӧ��֮��ĽӴ������С��
��3���ڹ�ҵ����ʯ��ʯ����ʯ�ң������ƣ��ķ�Ӧ����ʽΪ��CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2
���ø���Ʒʯ��ʯ125t����ʯ�ң���Ʒ�е����ʲ��ᷢ����Ӧ�����ʿ����Ƶú����ʵ���ʯ�������Ƕ��٣���Ҫ��д��������̣�
���� ��1���������ɶ�����̼�������뷴Ӧʱ��Ĺ�ϵͼ����Ʒ��ַ�Ӧ������̼���������淴Ӧʱ���ӳ������ӣ���ʱ���ö�����̼����Ϊ4.4g�������ɶ�����̼�����������ݷ�Ӧ�Ļ�ѧ����ʽ���ɼ������Ʒ��̼��Ƶ������������ɼ������Ʒ�е�̼��Ƶ�����������
��2���������ɶ�����̼�������뷴Ӧʱ��Ĺ�ϵͼ�е������ķ�ĩ״ʯ��ʯ���״ʯ��ʯ��ȫ��Ӧ������ʱ��Ĺ�ϵ���ƶ�Ӱ��û�ѧ��Ӧ���ʵ�������
��3������̼��Ƶ�������������Ƶ��������ɣ�
��� �⣺���ݹ�ϵͼ��֪����Ʒ��ַ�Ӧ�����ɶ�����̼������Ϊ4.4g
����Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
$\frac{100}{x}=\frac{44}{4.4g}$
x=10.0g
��Ʒ��̼��Ƶ���������Ϊ$\frac{10.0g}{12.5g}$��100%=80%��
���4.4��80%��
��2�������ɶ�����̼�������뷴Ӧʱ��Ĺ�ϵͼ����֪�������ķ�ĩ״��̼�����Ʒ��Ӧ���ٶȸ���һЩ����Ӧ���ĽӴ����Ӱ�컯ѧ��Ӧ���ٶȣ������Ӧ��֮��ĽӴ������С��
��3���⣺���Ƶ�CaO������Ϊx
CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2
100 56
125t��80% x
$\frac{100}{125t��80%}=\frac{56}{x}$
���x=56t
�������ʵ���ʯ��������56t+125t��1-80%��=81t
�𣺿��Ƶú������ʵ���ʯ������Ϊ81t��
���� �����ѶȲ������ո��ݻ�ѧ����ʽ�ļ��㼴����ȷ����⣬ϸ�µط������ɶ�����̼�������뷴Ӧʱ��Ĺ�ϵͼ��ȷ�����ɶ�����̼�������������ȷ�����Ĺؼ�

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�| A�� | ���ϻ� | B�� | ʳ�︯�� | C�� | ��ҵ������ | D�� | þ��ȼ�� |
| ���� | ���� | ��ȥ���ʵķ��� | |
| A | FeCl2��Һ | CuCl2 | �������������ۡ����� |
| B | CO2���� | CO | �õ�ȼ�ķ�����ȥ |
| C | ���� | ͭ�� | �ӹ���HCl��Һ������ |
| D | Cu | C | ͨ��O2���ȵ�ȼ |
| A�� | A | B�� | B | C�� | C | D�� | D |
����д�±��е�ʵ����ۣ�
| ʵ����� | ʵ������ | ʵ����� |
| ��ʢ������Fe2O3���Թ��м���NaCl��Һ���� | ���岻�ܽ� | Cl-����ʹFe2O3�ܽ⣬H2O����ʹFe2O3�ܽ⣬ʹ�������ܽ���������� |
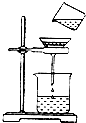 ��ͼ��С��ͬѧ�����ǵĺ�ˮ��Ʒ�����ձ��У��ȼ���������ĩ�����ܽ⣬����һ��������й��˵IJ�����
��ͼ��С��ͬѧ�����ǵĺ�ˮ��Ʒ�����ձ��У��ȼ���������ĩ�����ܽ⣬����һ��������й��˵IJ�����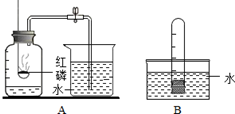 ijУ��ѧ��ȤС��Ϳ����������ĺ�������ʵ��̽����
ijУ��ѧ��ȤС��Ϳ����������ĺ�������ʵ��̽����