��Ŀ����
��������Ҫ�Ľ������ϣ�
��Ŀǰ����������50%���ϵķϾɸ����õ��������ã���Ŀ����________������ĸ��ţ���
A����Լ������Դ��B���������ɿ��C����ֹ��������
�ڸ߲㽨����¥����װ���ø������ı����룬�������ǽ������������£���˵�������������õ�________�ԣ���һ�������ʣ���
�۽����������Ʒ����ʢ��ϡ�����ϴ���У��۲쵽��Һ����ɫ��Ϊ��ɫ���˷�Ӧ�Ļ�ѧ����ʽ________��ʵ����ȡ��100mLŨ��Ϊ10%���ᣨ�ܶ�Ϊ1.1g/mL�������������������Ʒ��ȫ��Ӧ��ͬʱ������0.1g���壮��������������ⷴӦ�����ʵ�������________�ˣ�ʵ�ʳ�ȥ����������Fe2O3��������Ϊ________�ˣ������ݻ�ѧ����ʽ��ʽ���㣩
A ������ Fe2O3+6HCl=2FeCl3+3H2O 3.65 5.37
�������ٷϾɸ����������ÿ��Խ�ʡ������Դ��
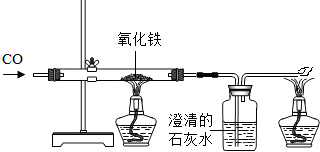
�ڱ����뽫�絼���������������ĵ����ԣ�
����Һ����ɫ��Ϊ��ɫ�����������������ᷴӦ�������Ȼ�����Ե�ʣ�ͬʱ������ˮ���ɴ˿�д���÷�Ӧ�Ļ�ѧ����ʽ��������ػ�ѧ����ʽ��������������������Լ����������Ӧ�����������ʵ������������õ������ⷴӦ�����������ʵ����������������ⷴӦ�����������ʵ���������������������
��𣺢ٷϾɸ����ǿ��������õ���Դ����˻������÷Ͼɸ������Խ�ʡ������Դ����ѡA��
�ڱ����뽫�絼���������������ĵ����ԣ����������ԡ���
����Һ����ɫ��Ϊ��ɫ�����������������ᷴӦ�������Ȼ�����Ե�ʣ�ͬʱ������ˮ�����ݻ�ѧ����ʽ����д��������д���÷�Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+6HCl=2FeCl3+3H2O��
��������Ӧ�����������ʵ�����Ϊx��
Fe+2HCl=FeCl2+H2��
73 2
x 0.1g
 =
= ��x=3.65g
��x=3.65g
�����ⷴӦ�����������ʵ�����Ϊ
1.1g/mL��100mL��10%-3.65g=7.35g
���ȥ������������Ϊy��
Fe2O3+6HCl=2FeCl3+3H2O
160 219
y 7.35g
 =
= ��y��5.37g
��y��5.37g
�ʴ�Ϊ����A���ڵ����ԣ���Fe2O3+6HCl=2FeCl3+3H2O��3.65g��5.37g��
������������Ҫ�����������֪ʶ�ͻ�ѧ����ʽ�ļ��㣬�ѶȽϴ�ͨ�������������ʺ������õ������Լ���Ļ�ѧ���ʲ��ܵó�ǰ���յĴ𰸣��ڽ��к����յĽ��ʱ������Ҫ������ֳ�������Ӧ�������ⷴӦ�������������֣�
�������ٷϾɸ����������ÿ��Խ�ʡ������Դ��
�ڱ����뽫�絼���������������ĵ����ԣ�
����Һ����ɫ��Ϊ��ɫ�����������������ᷴӦ�������Ȼ�����Ե�ʣ�ͬʱ������ˮ���ɴ˿�д���÷�Ӧ�Ļ�ѧ����ʽ��������ػ�ѧ����ʽ��������������������Լ����������Ӧ�����������ʵ������������õ������ⷴӦ�����������ʵ����������������ⷴӦ�����������ʵ���������������������
��𣺢ٷϾɸ����ǿ��������õ���Դ����˻������÷Ͼɸ������Խ�ʡ������Դ����ѡA��
�ڱ����뽫�絼���������������ĵ����ԣ����������ԡ���
����Һ����ɫ��Ϊ��ɫ�����������������ᷴӦ�������Ȼ�����Ե�ʣ�ͬʱ������ˮ�����ݻ�ѧ����ʽ����д��������д���÷�Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+6HCl=2FeCl3+3H2O��
��������Ӧ�����������ʵ�����Ϊx��
Fe+2HCl=FeCl2+H2��
73 2
x 0.1g
 =
= ��x=3.65g
��x=3.65g�����ⷴӦ�����������ʵ�����Ϊ
1.1g/mL��100mL��10%-3.65g=7.35g
���ȥ������������Ϊy��
Fe2O3+6HCl=2FeCl3+3H2O
160 219
y 7.35g
 =
= ��y��5.37g
��y��5.37g�ʴ�Ϊ����A���ڵ����ԣ���Fe2O3+6HCl=2FeCl3+3H2O��3.65g��5.37g��
������������Ҫ�����������֪ʶ�ͻ�ѧ����ʽ�ļ��㣬�ѶȽϴ�ͨ�������������ʺ������õ������Լ���Ļ�ѧ���ʲ��ܵó�ǰ���յĴ𰸣��ڽ��к����յĽ��ʱ������Ҫ������ֳ�������Ӧ�������ⷴӦ�������������֣�

��ϰ��ϵ�д�
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�
�����Ŀ

 ��������Ҫ�Ľ������ϣ��ڽ��컴����ˮ�ɻ���ʱ�������˴����ĸ�����
��������Ҫ�Ľ������ϣ��ڽ��컴����ˮ�ɻ���ʱ�������˴����ĸ�����