��Ŀ����
ij��ȤС����д����ᴿ������NaCl��Һ����ش��������⣺

��1�������١��ڡ�������Ϊͼ�еģ�����ĸ��________��b��________��
��2����NaCl�IJ���ƫ�ͣ� ����= ��100%��������ܵ�ԭ���ǣ�����ĸ��________��
��100%��������ܵ�ԭ���ǣ�����ĸ��________��
A������ʱ��ֽ�����𡡡�������B������ʱ�й��彦�� C���ܽ⺬����ɳ�Ĵ�ʳ��ʱ�������ˮ������
��3��������ʵ������NaCl��������8% NaCl��Һ60.0g���������²���˳����У�

��ش��������⣺
�ټ��㣺��Ҫ�Ȼ���________g��ˮ________g��
����������ƽ����������Ȼ���ʱ������������ƽ��ָ��ƫ�����̣�Ӧ________��
A�����������Ȼ��ƹ��塡����B�����������Ȼ��ƹ��� C������ƽ����ĸD���ƶ�����
�����в���������������Һ���Ȼ��Ƶ���������ƫС����________
A������Ͳ��ȡˮʱ���Ӷ�������������B��������Һʱ���ձ�������������ˮ��ϴ
C���Ȼ��ƾ��岻��������������������D��ת������õ���Һʱ����������Һ������
����Ϊͼ�����Ӧ��Ϊ��a��Һ��������b�����ˣ�c���ܽ⣮���Բ�������c��Ӧ����������b��Ӧ����������a��Ӧ��
��2����NaCl�IJ���ƫ�ͣ�����ԭ������ʱ�й��彦�����ܽ⺬����ɳ�Ĵ���ʱ�������ˮ����������ʱ�й��彦������ѡBC��
��3���ٸ������ʵ����������ļ��㹫ʽ����֪��������60.0g8% NaCl��Һ�����ʵ�����Ϊ��60.0g��8%=4.8g����ˮ������Ϊ��60.0g-4.8g=55.2g��
����������ƽ����������Ȼ���ʱ������������ƽ��ָ��ƫ�����̣����������������֪����������Ȼ���ƫ�࣬����Ӧ�ü����������Ȼ��ƹ��壬��ѡB��
��A������Ͳ��ȡˮʱ���Ӷ��������ʹ��ȡ��Һ��ƫ�Ӷ�ʹ���ʵ�����������С����A����
B��������Һʱ���ձ�������������ˮ��ϴ����ʹ�ܼ����������ӣ��Ӷ�ʹ���ʵ�����������С����B����
C���Ȼ��ƾ��岻������ʹ���ʵ�����ƫС���������Ƶ���Һ�����ʵ���������Ҳ��ƫС����C����
D����Һ���о�һ�Ժ��ȶ��ԣ�����ת������õ���Һʱ����������Һ����������Ӱ�����ʵ�������������D��ȷ��
��ѡD��
�ʴ�Ϊ����1��c��a��
��2��BC��
��3����4.8g��55.2g��
��BC��
��D��
��������1��������Ϊ�������ܽ⣻�����ں�����ɳ�Ļ��Һ�������ˣ��ýϴ������Ȼ�����Һ��������������Һ�����Ȼ��ƾ��壮
��2����NaCl�IJ���ƫ�ͣ�����ԭ������ʱ�й��彦�����ܽ⺬����ɳ�Ĵ�ʳ��ʱ�������ˮ�����㣬���Ծݴ˽��
��3���ٸ������ʵ����������ļ��㹫ʽ���м��㣻
�ڸ�����ƽ��ʹ�÷��������
�۸������ʵ����������ļ��㹫ʽ����֪��������������ƫС�����������ˣ������ܼ����ˣ����Ծݴ˽��
�����������ܽ⡢���ˡ�������ʵ��IJ������ܣ�������˷����������ԭ���������˵�ԭ�����������������ʵ���е�Ӧ�ã�

 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д���ȤС��ι�ij�Ƽ���������Ϣ���������������о���
���������ϡ�
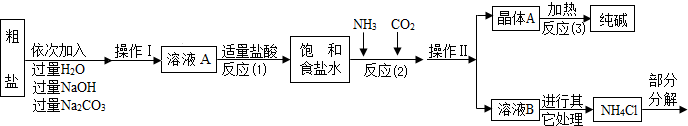
�ٴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
�ڷ�Ӧԭ����NaCl�����ͣ�+NH3+CO2+H2O=NaHCO3��+NH4Cl����������ľ���A��ּ��ȣ����Ƶô��
��NH4Cl NH3��+HCl����
NH3��+HCl����
����ˮ����ͭ��ˮ����
�ݲ���������������ͼ��ʾ��
���������ۡ�
��1����д����������������Һ��������Ӧ�Ļ�ѧ����ʽ______��
�ڲ����������Ϊ______��
�۷�Ӧ��1���м����������������______��
�ܷ�Ӧ��2����Ϊ��߲��ʣ����������˳����______������ĸ����
A����ͨ�������̼��ͨ�������� B����ͨ�백����ͨ������̼
��2���������������в���ѭ��ʹ�õ���______������ĸ����
A��CO2��������B��NH3��������C��HCl�������� D��NaOH
�����̽��һ��
��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ______��
�����ʵ����鴿����Ʒ���Ƿ���о���A������±���
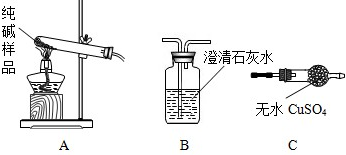
| ѡ���װ�� | ʵ������ | ʵ����� |
| ______ | ______ | ��Ʒ��������A |
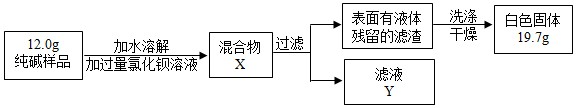
��4��ȡ������Ʒ��ˮ�ܽ⣬�����м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ______��ȷ��������Ʒ��������NaCl��
�����̽������
��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺

���жϼ���BaCl2��Һ�Ƿ�����ĺ��ʷ�����______���۲������жϣ�
A�����û����X�����ϲ���Һ���ٵ�BaCl2��Һ
B������ҺY�еμ�BaCl2��Һ
���ж������Ƿ�ϴ�Ӹɾ����ɲ�ȡ��ϴ��Һ�еμ�______���۲������жϣ�
A��BaCl2��Һ������ B��ϡH2SO4������C��Na2CO3��Һ����D��ϡHCl
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ______�� ��д��������̣�
��ȤС��ι�ij�Ƽ���������Ϣ���������������о���
���������ϡ�
�ٴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
�ڷ�Ӧԭ����NaCl�����ͣ�+NH3+CO2+H2O=NaHCO3��+NH4Cl����������ľ���A��ּ��ȣ����Ƶô��
��NH4Cl NH3��+HCl����
NH3��+HCl����
����ˮ����ͭ��ˮ����
�ݲ���������������ͼ��ʾ��

���������ۡ�
��1����д����������������Һ��������Ӧ�Ļ�ѧ����ʽ��������
�ڲ����������Ϊ����
�۷�Ӧ��1���м��������������������
�ܷ�Ӧ��2����Ϊ��߲��ʣ����������˳��������������ĸ����
A����ͨ�������̼��ͨ���� B����ͨ�백����ͨ������̼
��2���������������в���ѭ��ʹ�õ�������������ĸ����
A��CO2 B��NH3 C��HCl D��NaOH
�����̽��һ��
��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ��������
�����ʵ����鴿����Ʒ���Ƿ���о���A������±���

| ѡ���װ�� | ʵ������ | ʵ����� |
| ���� | �� | ��Ʒ��������A |
�����̽������
��4��ȡ������Ʒ��ˮ�ܽ⣬�����м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ����ȷ��������Ʒ��������NaCl��
�����̽������
��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺

���жϼ���BaCl2��Һ�Ƿ�����ĺ��ʷ������������۲������жϣ�
A�����û����X�����ϲ���Һ���ٵ�BaCl2��Һ
B������ҺY�еμ�BaCl2��Һ
���ж������Ƿ�ϴ�Ӹɾ����ɲ�ȡ��ϴ��Һ�еμ��������۲������жϣ�
A��BaCl2��Һ B��ϡH2SO4 C��Na2CO3��Һ D��ϡHCl
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ�� ��д��������̣�
���������ϡ�
�ٴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
�ڷ�Ӧԭ����NaCl�����ͣ�+NH3+CO2+H2O=NaHCO3��+NH4Cl����������ľ���A��ּ��ȣ����Ƶô��
��NH4Cl
 NH3��+HCl����
NH3��+HCl��������ˮ����ͭ��ˮ����
�ݲ���������������ͼ��ʾ��

���������ۡ�
��1����д����������������Һ��������Ӧ�Ļ�ѧ����ʽ ��
�ڲ����������Ϊ ��
�۷�Ӧ��1���м���������������� ��
�ܷ�Ӧ��2����Ϊ��߲��ʣ����������˳���� ������ĸ����
A����ͨ�������̼��ͨ���� B����ͨ�백����ͨ������̼
��2���������������в���ѭ��ʹ�õ��� ������ĸ����
A��CO2 B��NH3 C��HCl D��NaOH
�����̽��һ��
��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ ��
�����ʵ����鴿����Ʒ���Ƿ���о���A������±���

| ѡ���װ�� | ʵ������ | ʵ����� |
| ��Ʒ��������A |
��4��ȡ������Ʒ��ˮ�ܽ⣬�����м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ ��ȷ��������Ʒ��������NaCl��
�����̽������
��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺

���жϼ���BaCl2��Һ�Ƿ�����ĺ��ʷ����� ���۲������жϣ�
A�����û����X�����ϲ���Һ���ٵ�BaCl2��Һ
B������ҺY�еμ�BaCl2��Һ
���ж������Ƿ�ϴ�Ӹɾ����ɲ�ȡ��ϴ��Һ�еμ� ���۲������жϣ�
A��BaCl2��Һ B��ϡH2SO4 C��Na2CO3��Һ D��ϡHCl
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ ��д��������̣�
���������ϡ�
�ٴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
�ڷ�Ӧԭ����NaCl�����ͣ�+NH3+CO2+H2O=NaHCO3��+NH4Cl����������ľ���A��ּ��ȣ����Ƶô��
��NH4Cl
 NH3��+HCl����
NH3��+HCl��������ˮ����ͭ��ˮ����
�ݲ���������������ͼ��ʾ��

���������ۡ�
��1����д����������������Һ��������Ӧ�Ļ�ѧ����ʽ ��
�ڲ����������Ϊ ��
�۷�Ӧ��1���м���������������� ��
�ܷ�Ӧ��2����Ϊ��߲��ʣ����������˳���� ������ĸ����
A����ͨ�������̼��ͨ���� B����ͨ�백����ͨ������̼
��2���������������в���ѭ��ʹ�õ��� ������ĸ����
A��CO2 B��NH3 C��HCl D��NaOH
�����̽��һ��
��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ ��
�����ʵ����鴿����Ʒ���Ƿ���о���A������±���

| ѡ���װ�� | ʵ������ | ʵ����� |
| ��Ʒ��������A |
��4��ȡ������Ʒ��ˮ�ܽ⣬�����м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ ��ȷ��������Ʒ��������NaCl��
�����̽������
��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺

���жϼ���BaCl2��Һ�Ƿ�����ĺ��ʷ����� ���۲������жϣ�
A�����û����X�����ϲ���Һ���ٵ�BaCl2��Һ
B������ҺY�еμ�BaCl2��Һ
���ж������Ƿ�ϴ�Ӹɾ����ɲ�ȡ��ϴ��Һ�еμ� ���۲������жϣ�
A��BaCl2��Һ B��ϡH2SO4 C��Na2CO3��Һ D��ϡHCl
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ ��д��������̣�