��Ŀ����
��Ĺ������ͭ������һЩ����ɫ���ʣ��������׳ơ�ͭ�̡����仯ѧ���ΪCu2(OH)2CO3��С��ͬѧ�о�������ʱ��������ʵ�顣
��ʵ��һ�� ȡ2.22g ��ͭ�̡����壬�����м���������7.3%��ϡ���ᣬʹ������ȫ�ܽ⡣��Ӧ����ʽΪ�� Cu2(OH)2CO3+4HCl==2CuCl2+CO2��+3H2O������μӷ�Ӧ��ϡ�����������
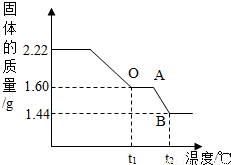
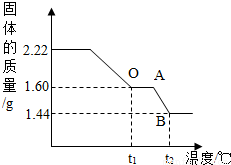
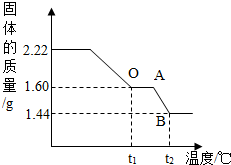
��ʵ�������1����ȡ2.22g��ͭ�̡��������ʹ��ֽ⣬���Ƴ�������������¶ȵı仯��ϵͼ����ͼ��t1��ʱȫ����Ϊ��ɫ���壬��˹��������ɵĶ�����̼��ˮ��������Ϊ_________��
��ʵ��һ�� ȡ2.22g ��ͭ�̡����壬�����м���������7.3%��ϡ���ᣬʹ������ȫ�ܽ⡣��Ӧ����ʽΪ�� Cu2(OH)2CO3+4HCl==2CuCl2+CO2��+3H2O������μӷ�Ӧ��ϡ�����������
��ʵ�������1����ȡ2.22g��ͭ�̡��������ʹ��ֽ⣬���Ƴ�������������¶ȵı仯��ϵͼ����ͼ��t1��ʱȫ����Ϊ��ɫ���壬��˹��������ɵĶ�����̼��ˮ��������Ϊ_________��
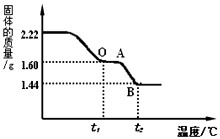
��2������ͼ�����㲢���������µ�t2���BC�β�������ĺ�����ѧʽ________��Ҫ���м�����̣���
�⣺��μӷ�Ӧ��ϡ���������Ϊx��
Cu2(OH)2CO3+4HCl==2CuCl2+CO2��+3H2O
����222��������������146
����2.22g����������7.3%x
222:2.22=146:7.3%x
��x = 20g
��ʵ�����(1)0.62g
��2���⣺����ͼ������֪����t1�浽t2�������������٣�1.60g-1.44g=0.16g
t1��ʱ1.60g����ͭ��ͭԪ������=1.60g��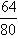 =1.28g
=1.28g
t1��ʱ1.60g����ͭ����Ԫ������=1.60g��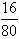 =0.32g
=0.32g
��ΪBC�ι�������Ԫ������Ϊ:0.32g-0.16g=0.16g������BC�ι�����ͭ����Ԫ��������=1.28g:0.16g=8:1
��BC�ι���Ļ�ѧʽΪCuxOy�� ��64x:16y=8:1��x ��y=2:1����Cu��OԪ��ԭ�Ӹ�����=2:1��
����BC��ʱ���廯ѧʽΪCu2O��
Cu2(OH)2CO3+4HCl==2CuCl2+CO2��+3H2O
����222��������������146
����2.22g����������7.3%x
222:2.22=146:7.3%x
��x = 20g
��ʵ�����(1)0.62g
��2���⣺����ͼ������֪����t1�浽t2�������������٣�1.60g-1.44g=0.16g
t1��ʱ1.60g����ͭ��ͭԪ������=1.60g��
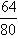 =1.28g
=1.28gt1��ʱ1.60g����ͭ����Ԫ������=1.60g��
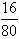 =0.32g
=0.32g��ΪBC�ι�������Ԫ������Ϊ:0.32g-0.16g=0.16g������BC�ι�����ͭ����Ԫ��������=1.28g:0.16g=8:1
��BC�ι���Ļ�ѧʽΪCuxOy�� ��64x:16y=8:1��x ��y=2:1����Cu��OԪ��ԭ�Ӹ�����=2:1��
����BC��ʱ���廯ѧʽΪCu2O��

��ϰ��ϵ�д�
�����Ŀ
 ������ɽ��Ĺ������ͭ������һЩ����ɫ���ʣ��������׳ơ�ͭ�̡����仯ѧ���ΪCu2��OH��2CO3��С��ͬѧ�о�������ʱ��������ʵ�飮
������ɽ��Ĺ������ͭ������һЩ����ɫ���ʣ��������׳ơ�ͭ�̡����仯ѧ���ΪCu2��OH��2CO3��С��ͬѧ�о�������ʱ��������ʵ�飮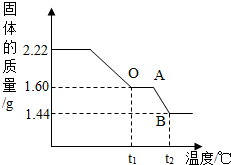 ������ɽ��Ĺ������ͭ������һЩ����ɫ���ʣ��������׳ơ�ͭ�̡����仯ѧ���ΪCu2��OH��2CO3��С��ͬѧ�о�������ʱ��������ʵ�飮
������ɽ��Ĺ������ͭ������һЩ����ɫ���ʣ��������׳ơ�ͭ�̡����仯ѧ���ΪCu2��OH��2CO3��С��ͬѧ�о�������ʱ��������ʵ�飮