��Ŀ����
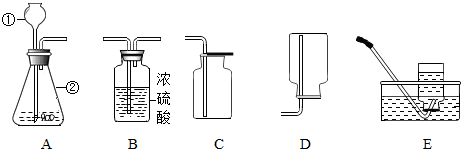
17��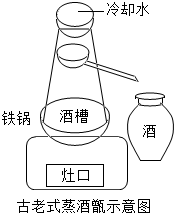 ��ͼ���ҹ�����ʽ�����ʾ��ͼ�������о����ﺬ�Ҵ���ˮ����Ʒ����������������ˮ���Ҵ��������������Ȳ��ú���������е��Ҵ�Ũ�Ƚϸߣ��������Ⱥ���Ũ�Ƚ��ͣ���ش�
��ͼ���ҹ�����ʽ�����ʾ��ͼ�������о����ﺬ�Ҵ���ˮ����Ʒ����������������ˮ���Ҵ��������������Ȳ��ú���������е��Ҵ�Ũ�Ƚϸߣ��������Ⱥ���Ũ�Ƚ��ͣ���ش���1��Ϊ��߹�Ч������������ǰҪ��������������еIJ������˲��������ǹ��ˣ�
��2����֪����ͬ�����£��Ҵ��е��ˮ�ͣ�������������������Ⱥ����Ҵ�Ũ�Ƚ��͵�ԭ�������������¶ȵIJ������ߣ�ˮ���ں�������Ȼ������������У�ʹ���е��Ҵ�Ũ�Ƚ��ͣ�Ϊ�õ�Ũ�Ƚϸߵľƣ���θ�����װ�ã���Ľ����ǿ��Ƽ����¶ȣ�ʹ�����ڵ��¶ȴﲻ��ˮ�ķе㣻
��3�����е�����Ϊ�ƾ�����ɫ���ľ��Ǵ����ﻹ�ǻ�������
��4��10mLˮ��10mL��ˮ�Ҵ���ϣ�������Һ���������20mL����������е�ԭ����Ӽ���һ���ļ����
��5���������в������10%���Ҵ���Ϊ���˵�����ȼ��---�Ҵ����ͣ���ȼ�����Ҵ�ȼ�յĻ�ѧ����ʽΪC2H5OH+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO2+3H2O��ʹ�ø�ȼ�ϲ��ֵؽ���˵�ǰ�������ٵ����⣬���һ�������Խ�Լ����������Դ������β������Ⱦ��
���� ��1�����ݹ��˲��������ú�ԭ��������
��2�������Ҵ���ˮ�ķе���з������
��3�����е������Ǿƾ���ѧ���Ҵ������ݴ���������ĸ�����з������
��4�����ݷ��Ӽ���һ���ļ�����з������
��5�����ݷ�Ӧ������P�����غ㶨��д����ѧ����ʽ���ɣ������Ҵ�ȼ��������Ի�����Ӱ����з������
��� �⣺��1����Ʒ��������������ˮ�����Կ����ù��˵ķ����Ѿ����еIJ������������
��2������ˮ�ķе�����Ҵ��ķе㣬���Կ�ʼ����ʱ�������ڵ��¶ȴﲻ��ˮ�ķе㣬�����ľ����Ҵ������ϸߣ������������¶ȵIJ������ߣ�ˮ���ں�������Ȼ������������У�ʹ���е��Ҵ�Ũ�Ƚ��ͣ�Ϊ�õ�Ũ�Ƚϸߵľƣ�����취���Ƽ����¶ȣ�ʹ�����ڵ��¶ȴﲻ��ˮ�ķе㣬����Ҳ�Ͳ���ʹˮ������������ˣ�
��3�����е������Ǿƾ���ѧ���Ҵ�����ɫ���ľ��к���ˮ���ƾ��ȶ������ʣ����ڻ���
��4����Ϊ���Ӽ���һ���ļ����10mLˮ��10mL��ˮ�Ҵ���ϣ�������Һ���������20mL��
��5���Ҵ�ȼ�տ����ɶ�����̼��ˮ���仯ѧ����ʽΪ��C2H5OH+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO2+3H2O��ʹ���Ҵ����͵ĺô����Ҵ������ǿ�������Դ�����Խ�Լ����������Դ������β������Ⱦ��
�ʴ�Ϊ����1�����ˣ���2�������������¶ȵIJ������ߣ�ˮ���ں�������Ȼ������������У�ʹ���е��Ҵ�Ũ�Ƚ��ͣ����Ƽ����¶ȣ�ʹ�����ڵ��¶ȴﲻ��ˮ�ķе㣻��3���ƾ���������4�����Ӽ���һ���ļ������5��C2H5OH+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO2+3H2O����Լ����������Դ������β������Ⱦ��
���� �����ʱҪ����˽��Ҵ������ʼ���ȼ��������Ի�����Ӱ�죬ֻ���������ܶ�����������ȷ���жϣ�

 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�| A�� | �������Ѿ� | B�� | �ϳ����� | C�� | ������ʴ | D�� | ������ˮ |
| ���� | �������� | ��ȥ���ʵķ��� | |
| A | CO���� | CO2���� | ͨ�������� ����������Һ |
| B | NaOH��Һ | Na2CO3 | ���������� ϡ���������ٲ������� |
| C | CaO���� | CaCO3 | ��ˮ�ܽ⣬���� |
| D | FeSO4��Һ | CuSO4 | ��������п������ַ�Ӧ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ʯī | B�� | ʳ�� | C�� | ˮ | D�� | ����̿ |