��Ŀ����
 ��ͼ���ҷֱ�Ϊ�������������ȼ�յ�ʾ��ͼ����ƿ����������ˮ���ش��������⣬
��ͼ���ҷֱ�Ϊ�������������ȼ�յ�ʾ��ͼ����ƿ����������ˮ���ش��������⣬��1���ֱ�д����������Ӧ�ķ��ű���ʽ��
��
S+O2
SO2
| ||
S+O2
SO2
��
| ||
3Fe+2O2
Fe3O4
| ||
3Fe+2O2
Fe3O4
| ||
3Fe+2O2
Fe3O4
| ||
3Fe+2O2
Fe3O4
��
| ||
��2����ƿ��ˮ�����÷ֱ��ǣ�
��
�������ɵ��ж����壬��ֹ��Ⱦ����
�������ɵ��ж����壬��ֹ��Ⱦ����
����ֹ���ɵ��������ヲ��ƿ��
��ֹ���ɵ��������ヲ��ƿ��
������ƿը��
������ƿը��
����������1����������������ȼ�����ɶ��������Լ���˿��������ȼ�������������������н�𣻣�2�����ݼ���ƿ�ײ�����ˮ��ɵĺ�����ǣ�
����⣺��1������������ȼ�����ɶ�������Ӧ�ķ��ű���ʽS+O2
SO2��
��˿��������ȼ��������������������Ӧ�ķ��ű���ʽ3Fe+2O2
Fe3O4��
��2�����ڶ��������ж����ڿ�����Ⱦ�������ˮ�����ն�������ֹ��Ⱦ��������˿ȼ�յ��������¶Ⱥܸ����ֱ�ӵ�������ƿ�ײ�����ը��ƿ�ף����Է�ˮ��Ϊ�˷�ֹ���ɵ��������ヲ��ƿ�ף�������ƿը�ѣ���
�ʴ�Ϊ����1��S+O2
SO2��3Fe+2O2
Fe3O4��
��2���������ɵ��ж����壬��ֹ��Ⱦ��������ֹ���ɵ��������ヲ��ƿ�ף�������ƿը�ѣ�
| ||
��˿��������ȼ��������������������Ӧ�ķ��ű���ʽ3Fe+2O2
| ||
��2�����ڶ��������ж����ڿ�����Ⱦ�������ˮ�����ն�������ֹ��Ⱦ��������˿ȼ�յ��������¶Ⱥܸ����ֱ�ӵ�������ƿ�ײ�����ը��ƿ�ף����Է�ˮ��Ϊ�˷�ֹ���ɵ��������ヲ��ƿ�ף�������ƿը�ѣ���
�ʴ�Ϊ����1��S+O2
| ||
| ||
��2���������ɵ��ж����壬��ֹ��Ⱦ��������ֹ���ɵ��������ヲ��ƿ�ף�������ƿը�ѣ�
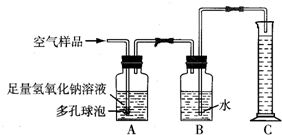
������ͨ���ش���֪������˿ȼ�ա���ȼ�յ�ע���������������ȼ�յ�����ֹ���ڴ��������ɼ���ƿը�ѺͿ�����Ⱦ������

��ϰ��ϵ�д�
�����Ŀ
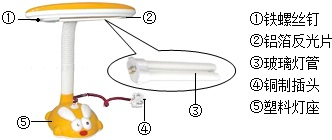 ��2013?��������ģ���������������������й㷺��Ӧ�ã�
��2013?��������ģ���������������������й㷺��Ӧ�ã�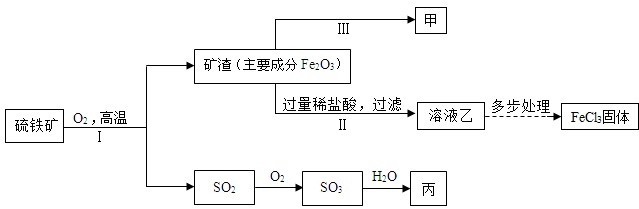
 A��ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ֻҪ����������꣬�Ը�����ɼ����ƻ���
A��ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ֻҪ����������꣬�Ը�����ɼ����ƻ��� ��ͼ���ҷֱ�Ϊ�������������ȼ�յ�ʾ��ͼ����ƿ����������ˮ���ش��������⣬
��ͼ���ҷֱ�Ϊ�������������ȼ�յ�ʾ��ͼ����ƿ����������ˮ���ش��������⣬