��Ŀ����
Ϊ�˴ﵽʵ��Ŀ�ģ��й�ʵ�鷽��������ǣ� ��

A. A B. B C. C D. D
A ��������A�����������ϡ���ᣬϡ���������ⷴӦ���Ժ�������������Ӧ������B����ȼ�յ�ľ�����뼯��ƿ�У����ȼ�յ�ľ��ȼ�ո�����������������������ȼ�յ�ľ��Ϩ�����Ƕ�����̼����ȷ��C����ȥ���ʵ�ԭ���������Լ�ֻ�����ʷ�Ӧ���Ҳ������µ����ʣ�CuO+CO ��Cu+CO2 ����ȷ��D�����������ܽ���ˮ�ų��������ȣ��Ȼ����ܽ���ˮ�����仯�����ԣ���ȷ����ѡA��
��ϰ��ϵ�д�
�����Ŀ


 ����ͭ������ͭ�ǹ��壩
����ͭ������ͭ�ǹ��壩
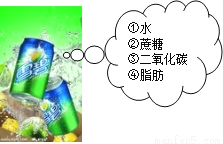


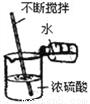 B.
B. 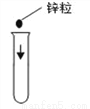 C.
C. 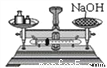 D.
D.